��Ŀ����
�����Ǽ���ʵ���г��õ�������

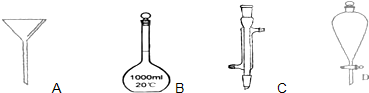
��1��д��ͼ1��������������������ƣ�B
��2��ͼ2��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ�
���ø�Ũ��������100mL��1mol/L��ϡ���ᣮ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
������ϡ����ʱ�����������в���Ҫʹ�õ���
���㣬��Ũ��������ʵ���Ũ��Ϊ
���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ������ķ�����
A����������Һ�壬ʹ��Һ����̶�������
B��С�ļ�������ƿ����������ʹ��Һ����̶�������
C�����������һ������Ũ����
D����������
���ø�Ũ��������100mL1mol/Lϡ�������ȷ˳����
A����ȴ B����ȡ C��ϴ�� D������ E���ܽ� F��ҡ�� G����Һ
���������Ƶ�ϡ������вⶨ������Ũ�ȴ���1mol/L������������ƹ����п������������ԭ���ǣ�����ţ�
������ƿ�����������������ˮ �ڶ���ʱ���ӿ̶��� �۶���ʱ���ӿ̶���
��δ��ȴ�����¾�ת�Ƶ�����ƿ�� ��û��ϴ���ձ��Ͳ�����
����д�������ݡ��ľ��������

��1��д��ͼ1��������������������ƣ�B
1000ml����ƿ
1000ml����ƿ
��C������
������
��D��Һ©��
��Һ©��
����2��ͼ2��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ�
���ø�Ũ��������100mL��1mol/L��ϡ���ᣮ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
������ϡ����ʱ�����������в���Ҫʹ�õ���
�ڢܢ�
�ڢܢ�
��ѡ����ţ�����ȱ�ٵ�������100mL����ƿ��������
100mL����ƿ��������
��д�������ƣ������㣬��Ũ��������ʵ���Ũ��Ϊ
18.4
18.4
mol/L������100mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ5.4
5.4
mL������һλС��������ȡŨ����ʱӦѡ����
��
��ѡ���10mL����50mL����100mL��������Ͳ�����ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ������ķ�����
D
D
������ţ���A����������Һ�壬ʹ��Һ����̶�������
B��С�ļ�������ƿ����������ʹ��Һ����̶�������
C�����������һ������Ũ����
D����������
���ø�Ũ��������100mL1mol/Lϡ�������ȷ˳����
BEAGCGDF
BEAGCGDF
��A����ȴ B����ȡ C��ϴ�� D������ E���ܽ� F��ҡ�� G����Һ
���������Ƶ�ϡ������вⶨ������Ũ�ȴ���1mol/L������������ƹ����п������������ԭ���ǣ�����ţ�
�ڢ�
�ڢ�
��������ƿ�����������������ˮ �ڶ���ʱ���ӿ̶��� �۶���ʱ���ӿ̶���
��δ��ȴ�����¾�ת�Ƶ�����ƿ�� ��û��ϴ���ձ��Ͳ�����
����д�������ݡ��ľ��������
����ʱ�IJ����ǣ���ˮ��Һ��������ƿ���̶�����1-2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶�������
����ʱ�IJ����ǣ���ˮ��Һ��������ƿ���̶�����1-2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶�������
����������1�����������������
��2������ݸ�����������ѡȡ������
��C=
������ϡ��ǰ�����ʵ����ʵ���������㣬����Ũ��������ѡȡ��Ͳ�Ĺ��
���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ�Ӧ���������ƣ�
������ʱ��һ��ɷ�Ϊ���¼������裺���㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ��ݴ˽�������
������C=
�жϣ�
������ʱ�IJ����ǣ���ˮ��Һ��������ƿ���̶�����1-2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
��2������ݸ�����������ѡȡ������
��C=
| 103��w |
| M |
���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ�Ӧ���������ƣ�
������ʱ��һ��ɷ�Ϊ���¼������裺���㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ��ݴ˽�������
������C=
| n |
| V |
������ʱ�IJ����ǣ���ˮ��Һ��������ƿ���̶�����1-2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
����⣺��1��ͼ�и������������ǣ�BΪ1000ml����ƿ��CΪ�����ܣ�DΪ��Һ©�����ʴ�Ϊ��1000ml����ƿ�������ܣ���Һ©����
��2��������ϡ����ʱ�����������в���Ҫʹ�õ��Тڢܢޣ�����Ҫ�������У�������Һ������100mL����ƿ���������ͽ������õIJ��������ʴ�Ϊ���ڢܢޣ�100mL����ƿ����������
��C=
=
mol/L=18.4mol/L��ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪV������
18.4 mol/L��V=1mol/L��0.1L��V=0.0054L=5.4mL������Ӧ��ѡȡ10mL��Ͳ��
�ʴ�Ϊ��18.4��5.4���٣�
���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ�Ӧ���������ƣ���ѡD��
������ʱ��һ��ɷ�Ϊ���¼������裺���㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�����������˳��Ϊ��BEAGCGDF���ʴ�Ϊ��BEAGCGDF��
��������ƿ�����������������ˮ�����ʵ����ʵ�������Һ����������䣬������Ӱ�죻
�ڶ���ʱ���ӿ̶��ߣ���Һ�����ƫС��������Һ��Ũ��ƫ��
�۶���ʱ���ӿ̶��ߣ���Һ�����ƫ��������Һ��Ũ��ƫС��
��δ��ȴ�����¾�ת�Ƶ�����ƿ�У���Һ�����ƫС��������Һ��Ũ��ƫ��
��û��ϴ���ձ��Ͳ����������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
��ѡ�ڢܣ�
������ʱ�IJ����ǣ���ˮ��Һ��������ƿ���̶�����1-2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
�ʴ�Ϊ����ˮ��Һ��������ƿ���̶�����1-2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
��2��������ϡ����ʱ�����������в���Ҫʹ�õ��Тڢܢޣ�����Ҫ�������У�������Һ������100mL����ƿ���������ͽ������õIJ��������ʴ�Ϊ���ڢܢޣ�100mL����ƿ����������
��C=
| 103��w |
| M |
| 103��1.84��98% |
| 98 |
18.4 mol/L��V=1mol/L��0.1L��V=0.0054L=5.4mL������Ӧ��ѡȡ10mL��Ͳ��
�ʴ�Ϊ��18.4��5.4���٣�
���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ�Ӧ���������ƣ���ѡD��
������ʱ��һ��ɷ�Ϊ���¼������裺���㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�����������˳��Ϊ��BEAGCGDF���ʴ�Ϊ��BEAGCGDF��
��������ƿ�����������������ˮ�����ʵ����ʵ�������Һ����������䣬������Ӱ�죻
�ڶ���ʱ���ӿ̶��ߣ���Һ�����ƫС��������Һ��Ũ��ƫ��
�۶���ʱ���ӿ̶��ߣ���Һ�����ƫ��������Һ��Ũ��ƫС��
��δ��ȴ�����¾�ת�Ƶ�����ƿ�У���Һ�����ƫС��������Һ��Ũ��ƫ��
��û��ϴ���ձ��Ͳ����������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
��ѡ�ڢܣ�
������ʱ�IJ����ǣ���ˮ��Һ��������ƿ���̶�����1-2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
�ʴ�Ϊ����ˮ��Һ��������ƿ���̶�����1-2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
���������⿼��һ�����ʵ���Ũ����Һ�����ƣ�ע������ƿ����Ͳ����ѡȡ������Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ


 B.
B. C.
C. D.
D.

 A
A  B
B  C
C  D
D