��Ŀ����
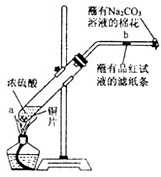
��12�֣�ijѧ����Ũ�������ʵ�ʵ�飺��һ֧�Թ��з���һ���С��ͭƬ���ټ���2mLŨ���ᣬȻ����Թ̶ܹ�������̨�ϡ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������С������Թܿڣ��ڲ����ܿڴ�����һ��պ��Na2CO3��Һ�����������Թܣ��۲�����.�ش��������⣺

��1��д���Թ��з�����Ӧ�Ļ�ѧ����ʽ ��
��2���Թ��е�Һ�巴Ӧһ��ʱ���b����ֽ���ı仯Ϊ �����Թ��з�Ӧֹͣ�������ܷ���պ��Ʒ����Һ����ֽ�����ȣ���ֽ���ı仯Ϊ ��
��3��պ��Na2CO3��Һ������������ ��
��4��������������γɹ��̿������з�Ӧ�е� ����ʾ��

��5��Ũ������������Ҫ���ʣ����뺬��ˮ�ֵ��������ù����в�����ʾ��������
A������ B����ˮ�� C��ǿ������ D����ˮ��
��12�֣���1��Cu+2H2SO4(Ũ)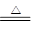 CuSO4+ SO2��+2H2O
CuSO4+ SO2��+2H2O
��2��պ��Ʒ����Һ����ֽ��ɫ ����ֽ���
��3�����ն���SO2����ֹ��Ⱦ���� ��4��A B ��5��A
��������
�����������1���ڼ��ȵ������£�Ũ�����ͭ��Ӧ�Ļ�ѧ����ʽ��Cu+2H2SO4(Ũ)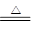 CuSO4+ SO2��+2H2O��
CuSO4+ SO2��+2H2O��
��2������Ļ�ԭ����SO2����Ư���ԣ���ʹƷ����Һ��ɫ����SO2��Ư���Dz��ȶ��ģ��ڼ��ȵ�����£����Իָ�����������ɫ��������ֽ���ָֻ���ɫ��
��3��SO2�Ǵ�����Ⱦ����պ��Na2CO3��Һ���������������ն���SO2����ֹ��Ⱦ������
��4�������е�SO2����������SO3��SO3����ˮ�������ᣬ���γ����꣬��ѡAB��
��5��Ũ���������ˮ�ԡ���ˮ�Ժ�ǿ�����ԡ�����ʹ����̿����������������CO2��SO2��ˮ�������ڸñ仯�����У�û��������������ԣ���ѡA��
���㣺����Ũ��������ʡ�SO2�ļ����β������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ���������У������ڿ���ѧ��������Ҳ����������ѧ����ʵ��̽���������Լ�������������ͬʱҲ����������ѧ���Ļ���������ʶ����ǿѧ����������θС�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� ijѧ����Ũ�������ʵ�ʵ�飺
ijѧ����Ũ�������ʵ�ʵ�飺

 ijѧ����Ũ�������ʵ�ʵ�飺
ijѧ����Ũ�������ʵ�ʵ�飺
