��Ŀ����
��16�֣���1��ij��A 0.2 mol�������г��ȼ�պ����ɻ�����B��C��1 mol��
����A�ķ���ʽΪ ��
����ȡһ������A��ȫȼ�պ�����B��C��3 mol������ g��A�μ��˷�Ӧ��
������A����ʹ��ˮ��ɫ������һ�������£�����Cl2����ȡ����Ӧ����һ�ȴ���ֻ��һ�֣���A�Ľṹ��ʽΪ ��
������A��ʹ��ˮ��ɫ���ڴ�����������H2�ӳɣ���ӳɲ�������к���3��������A���ܵĽṹ��ʽΪ ��
������һ�ַ����У�����̼ԭ�Ӷ���ͬһƽ���ڣ��������Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ ��
��2����������CH3CH3����CH2��CH2����CH3CH2C��CH����CH3C��CCH3����C2H6����CH3CH��CH2��
һ����Ϊͬϵ����� ��
һ����Ϊͬ���칹����� �������ţ�
��C5H10����42 (3��) ��
�� ��
�� ��
��
 ��2���ڢޣ��ۢܡ�
��2���ڢޣ��ۢܡ�
���������������1��������0.3mol�����к���C��1mol������H2mol,����1mol����A����C��5mol,����H10mol,����A�ķ���ʽΪC5H10������ȡһ������A��ȫȼ�պ�����B��C��3 mol�������Ԫ���غ��֪������Ӧ��A�����ʵ���Ϊ3 mol��5=0.6mol,��������0.6mol��70g/mol=42g��������A����ʹ��ˮ��ɫ������һ�������£�����Cl2����ȡ����Ӧ����һ�ȴ���ֻ��һ�֣���A�ǻ����飬�ṹ��ʽΪ ��������A��ʹ��ˮ��ɫ���ڴ�����������H2�ӳɣ���ӳɲ�������к���3��������A���ܵĽṹ��ʽΪ
��������A��ʹ��ˮ��ɫ���ڴ�����������H2�ӳɣ���ӳɲ�������к���3��������A���ܵĽṹ��ʽΪ ��
�� ��
�� ��������һ�ַ����У�����̼ԭ�Ӷ���ͬһƽ���ڣ��������Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ
��������һ�ַ����У�����̼ԭ�Ӷ���ͬһƽ���ڣ��������Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ ����2��ͬϵ���ǽṹ���ƣ��ڷ�����������һ�������ɸ�CH2ԭ���ŵĻ����һ����Ϊͬϵ����Ǣڢޣ�ͬ���칹���Ǿ�����ͬ�ķ���ʽ����ͬ�Ľṹ���л������һ����Ϊͬ���칹���Ǣۢܡ�
����2��ͬϵ���ǽṹ���ƣ��ڷ�����������һ�������ɸ�CH2ԭ���ŵĻ����һ����Ϊͬϵ����Ǣڢޣ�ͬ���칹���Ǿ�����ͬ�ķ���ʽ����ͬ�Ľṹ���л������һ����Ϊͬ���칹���Ǣۢܡ�
���㣺�������ʵķ���ʽ���ṹ��ȷ����ͬϵ�ͬ���칹��ĸ���ı������жϵ�֪ʶ��

���й���ʵ���������������ȷ����
| A����ʢ�б����Թ��м��뼸������KMnO4��Һ���ɹ۲쵽��ɫ��ȥ |
| B����ǿ������ʢ��CH4��Cl2(�����1��4)�ļ���ƿ�ɹ۲쵽ƿ�ڱڸ�����״�� |
| C����ֲ���͡��Ҵ���NaOH��Һ��һ����ȷ��ڷ�Ӧ�����м��ȡ����裬����Ӧ��ɺ��ټӷ��ȵı���ʳ��ˮ���ɹ۲쵽�����ײ��й����������� |
| D�������ȵ�ͭ˿Ѹ�ٲ����Ҵ��У�������Σ��ɹ۲쵽ͭ˿�����ڣ������ŵ���ζ |
���и�����������ͬ���칹����ǣ� ��
| A�����ۺ���ά�� |
B��CH3��CH2��CH2��CH3�� |
| C��1H ��2H |
| D��O2��O3 |
��10�֣�������ֲ��֦Ҷ��ȡ�ľ����к������мס������ֳɷ֣�
| ����ʽ | C9H8O |
| �������� | ��ʹBr2/CCl4��ɫ |

�� ��
��1���������������ŵ�����Ϊ��������������������
��2���ɼ�ת��Ϊ���辭���й���(����ȥ������Ӧ���ز����ͬ)��

���з�Ӧ��ķ�Ӧ����Ϊ_________��Y�Ľṹ��ʽΪ_________________��
��3���������������ҷ�Ӧ��������������ѡ����ţ���
a�� ������ b�� ��ˮ c�� ̼������Һ d�� ����
��4�����ж���ͬ���칹�壬д��ͬʱ��������������ͬ���칹��ṹ��ʽ ��
a�� �����ϵ�һ�ȴ��������� b�� ����������Ӧ
��14�֣���ҩ����dz��������ҩ�����Ч�ɷ�Ϊ�������Ԫ���������Ƹ�Ѫѹ�����ͷ�ۡ�ͷ�Ρ�ͻ���Զ�����֢����ϳ���·���£�
��֪��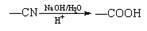 ��
�� 
��ش��������⣺
��1������C�Ľṹ��ʽΪ
��2�����ڸ������Ԫ������˵����ȷ����
| A��1 mol�������Ԫ�����Ժ�2 molNaOH��Ӧ | B��������������Ӧ |
| C���ɷ���ˮ�ⷴӦ | D������ˮ�ɷ����ӳɻ�ȡ����Ӧ |
��4������B�������۷�Ӧ���ɸ߷��ӻ�����ķ�Ӧ����ʽΪ
��5��д��ͬʱ������������������Cͬ���칹��Ľṹ��ʽ ��дһ�֣�
i��������Fe3+������ɫ��Ӧ ii�� ���Է���������Ӧ
iii�������������ֲ�ͬ��ѧ��������ԭ��
��6�������ºϳ���·��

������ѧ֪ʶ�������Ϣ�����������ϳ���·�ĺ������ϴ��ڵ�����



