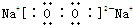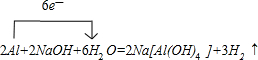��Ŀ����
D��EΪ��ѧ��ѧ�����Ľ������ʣ�F��һ�ֺ�ɫ���塣E��C��Ũ��Һ�ڳ�������������������ʱ���д�����ɫ���������ͬʱ����H����һ��������C��D���ܷ�����Ӧ��������ת����ϵ����ͼ��
 (1)B��ͬ�������������Ϊ_______ __��F�Ļ�ѧʽΪ_____ ___ __��
(1)B��ͬ�������������Ϊ_______ __��F�Ļ�ѧʽΪ_____ ___ __��
(2)д��D��C��Ũ��Һ��Ӧ�Ļ�ѧ����ʽ ��
(3)�ö��Ե缫���A ��Һ��������ӦʽΪ________ _ __ _��
(4)����H��G�Ļ����Һ�к���G�������ӵ��Լ�������_____ ____��
a.��ˮ��KSCN��Һ b.����������Һ c. ����KMnO4��Һ
(5)�ö��Ե缫���һ��Ũ�ȵ�A��Һ��ͨ��һ��ʱ�����������Һ�м���9.8g��A�н��������ӵ���������ǡ�ûָ������ǰ��Ũ�Ⱥ�pH�����������ת�Ƶ��ӵ����ʵ���Ϊ_____________mol, �ռ�����״���µ��������Ϊ_____________��
(1)���� (2��) Fe3O4 (2��)
(2) Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O��2�֣�
(3)4OH����4e����2H2O ��O2��(2��) (4)c (2��)
(5)0.4mol (2��) 4.48L(2��)
����:��

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д� A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش� A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ��ʾ��
A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ��ʾ��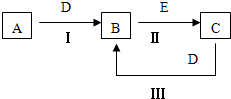 A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ��ʾ��
A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ��ʾ��