��Ŀ����
��2010?���ϣ�I��֪�� �����Ҫ�ϳ�
�����Ҫ�ϳ� ���õ�ԭʼԭ�Ͽ�����
���õ�ԭʼԭ�Ͽ�����
A.2-��-l��3-����ϩ��2-��Ȳ B��1��3-���ϩ��2-��Ȳ
C��2��3-����-1��3-���ϩ����Ȳ D����3-����-l��3-����ϩ�ͱ�Ȳ
II��A��G�����л���������ǵ�ת����ϵ���£�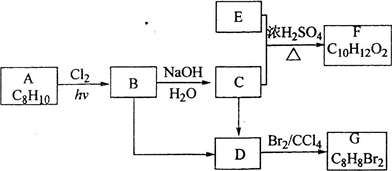
��ش��������⣺
��1����֪��6.0g������E��ȫȼ������8.8g C02��3.6g H20��E������������������ܶ�Ϊ30����E�ķ���ʽΪ
��2��AΪһȡ��������B�к���һ��������B����C�Ļ�ѧ����ʽΪ
��3����B����D����C����D�ķ�Ӧ�����ֱ���
��4����A����B����D����G�ķ�Ӧ���ͷֱ���
��5��F���������������У���ṹ��ʽΪ
 ��
��
��6����G��ͬ���칹���У�������һ�����IJ���ֻ��һ�ֵĹ���
 ����ṹ��ʽ����
����ṹ��ʽ����
 �����Ҫ�ϳ�
�����Ҫ�ϳ� ���õ�ԭʼԭ�Ͽ�����
���õ�ԭʼԭ�Ͽ�����AD
AD
A.2-��-l��3-����ϩ��2-��Ȳ B��1��3-���ϩ��2-��Ȳ
C��2��3-����-1��3-���ϩ����Ȳ D����3-����-l��3-����ϩ�ͱ�Ȳ
II��A��G�����л���������ǵ�ת����ϵ���£�

��ش��������⣺
��1����֪��6.0g������E��ȫȼ������8.8g C02��3.6g H20��E������������������ܶ�Ϊ30����E�ķ���ʽΪ
C2H4O2
C2H4O2
����2��AΪһȡ��������B�к���һ��������B����C�Ļ�ѧ����ʽΪ
C6H5CHClCH3+H2O
C6H5CHOHCH3+HCl
| NaOH |
| �� |
C6H5CHClCH3+H2O
C6H5CHOHCH3+HCl
��| NaOH |
| �� |
��3����B����D����C����D�ķ�Ӧ�����ֱ���
NaOH����Һ������
NaOH����Һ������
��Ũ���Ტ����
Ũ���Ტ����
����4����A����B����D����G�ķ�Ӧ���ͷֱ���
ȡ����Ӧ
ȡ����Ӧ
���ӳɷ�Ӧ
�ӳɷ�Ӧ
����5��F���������������У���ṹ��ʽΪ


��6����G��ͬ���칹���У�������һ�����IJ���ֻ��һ�ֵĹ���
7
7
�������к˴Ź�������������壬�ҷ������Ϊl��1����

���������Բ�������ϳɷ�����ȥ������ԭ�ϣ�
���ݰ����ӵ����ɵ���������ܶ�֮�ȵ���Ħ������֮�ȣ�E������������������ܶ�Ϊ30��Ħ��������60���������ɶ�����̼��ˮ������ȷ�����ʽ�����Ħ��������ȷ���л���ΪC2H4O2��Ϊ���ᣬ�ɿ�ͼ��֪E��C��������F����ԭ���غ�֪C�ķ���ʽ��C8H10O����ΪAΪһȡ����������B�к���һ����������B�Ľṹ��ʽΪC6H5CHClCH3��D������Br2�����ӳɷ�Ӧ������D�DZ���ϩ���ṹ��ʽΪC6H5CH=CH2��C��C6H5CHOHCH3�������������Ľṹ��ʽ�� ����������Ϣ���л���Ľṹ�����ʿɽ����⣮
����������Ϣ���л���Ľṹ�����ʿɽ����⣮
���ݰ����ӵ����ɵ���������ܶ�֮�ȵ���Ħ������֮�ȣ�E������������������ܶ�Ϊ30��Ħ��������60���������ɶ�����̼��ˮ������ȷ�����ʽ�����Ħ��������ȷ���л���ΪC2H4O2��Ϊ���ᣬ�ɿ�ͼ��֪E��C��������F����ԭ���غ�֪C�ķ���ʽ��C8H10O����ΪAΪһȡ����������B�к���һ����������B�Ľṹ��ʽΪC6H5CHClCH3��D������Br2�����ӳɷ�Ӧ������D�DZ���ϩ���ṹ��ʽΪC6H5CH=CH2��C��C6H5CHOHCH3�������������Ľṹ��ʽ��
 ����������Ϣ���л���Ľṹ�����ʿɽ����⣮
����������Ϣ���л���Ľṹ�����ʿɽ����⣮����⣺������Բ�������ϳɷ�������  ������
������ �������л��������ԭ������ԭ�Ϸֱ���2��3-����-l��3-����ϩ�ͱ�Ȳ������2-��-l��3-����ϩ��2-��Ȳ���ʴ�Ϊ��AD��
�������л��������ԭ������ԭ�Ϸֱ���2��3-����-l��3-����ϩ�ͱ�Ȳ������2-��-l��3-����ϩ��2-��Ȳ���ʴ�Ϊ��AD��
��1�����ݰ����ӵ����ɵ���������ܶ�֮�ȵ���Ħ������֮�ȣ����EĦ��������60.6.0gE�����ʵ�������0.1mol����ȫȼ�պ�����C02�� H20�����ʵ����ֱ�Ϊ
=0.2mol��
=0.2mol������̼����������ֱ�Ϊ0.2��12=2.4g��0.4��1=0.4g�����E����Ԫ�ص�����Ϊ6.0-2.4-0.4=3.2g��������Ԫ�ص����ʵ���Ϊ
=0.2mol�����̼���⡢������ԭ�Ӹ���֮��Ϊ1��2��1�������ʽΪCH2O����ΪEĦ��������60�����Է���ʽ��C2H4O2��
�ʴ�Ϊ��C2H4O2��
��2���ɿ�ͼ��֪E��C��������F����ԭ���غ�֪C�ķ���ʽ��C8H10O����ΪAΪһȡ����������B�к���һ����������B�Ľṹ��ʽΪC6H5CHClCH3��B����C�Ļ�ѧ����ʽ�ǣ�C6H5CHClCH3+H2O
C6H5CHOHCH3+HCl��
�ʴ�Ϊ��C6H5CHClCH3+H2O
C6H5CHOHCH3+HCl��
��3����ΪD������Br2�����ӳɷ�Ӧ������D�DZ���ϩ���ṹ��ʽΪC6H5CH=CH2��±����������ȥ��Ӧ��������NaOH����Һ�����ȣ���������ȥ��Ӧ��������Ũ���Ტ���ȣ�
�ʴ�Ϊ��NaOH����Һ�����ȣ�Ũ���Ტ���ȣ�
��4��A���ڱ���ͬϵ�B����±������������A����B�ķ�Ӧ������ȡ����Ӧ��D�к���̼̼˫���������D����G�ķ�Ӧ�����Ǽӳɷ�Ӧ���ʴ�Ϊ��ȡ����Ӧ���ӳɷ�Ӧ��
��5����ΪC��E���Է���������Ӧ������E�����ᣬ����ΪC��C6H5CHOHCH3�������������Ľṹ��ʽ�� ��
��
�ʴ�Ϊ�� ��
��
��6��������һ�����IJ���ֻ��һ�֣�˵��Ӧ���ǶԳ��Խṹ������G�ķ���ʽC8H8Br2��֪���������Ĺ�������7�֣�
���к˴Ź�������������壬�ҷ������Ϊl��1���� ��
��
�ʴ�Ϊ��7�� ��
��
 ������
������ �������л��������ԭ������ԭ�Ϸֱ���2��3-����-l��3-����ϩ�ͱ�Ȳ������2-��-l��3-����ϩ��2-��Ȳ���ʴ�Ϊ��AD��
�������л��������ԭ������ԭ�Ϸֱ���2��3-����-l��3-����ϩ�ͱ�Ȳ������2-��-l��3-����ϩ��2-��Ȳ���ʴ�Ϊ��AD����1�����ݰ����ӵ����ɵ���������ܶ�֮�ȵ���Ħ������֮�ȣ����EĦ��������60.6.0gE�����ʵ�������0.1mol����ȫȼ�պ�����C02�� H20�����ʵ����ֱ�Ϊ
| 8.8 |
| 44 |
| 3.6 |
| 18 |
| 3.2 |
| 16 |
�ʴ�Ϊ��C2H4O2��
��2���ɿ�ͼ��֪E��C��������F����ԭ���غ�֪C�ķ���ʽ��C8H10O����ΪAΪһȡ����������B�к���һ����������B�Ľṹ��ʽΪC6H5CHClCH3��B����C�Ļ�ѧ����ʽ�ǣ�C6H5CHClCH3+H2O
| NaOH |
| �� |
�ʴ�Ϊ��C6H5CHClCH3+H2O
| NaOH |
| �� |
��3����ΪD������Br2�����ӳɷ�Ӧ������D�DZ���ϩ���ṹ��ʽΪC6H5CH=CH2��±����������ȥ��Ӧ��������NaOH����Һ�����ȣ���������ȥ��Ӧ��������Ũ���Ტ���ȣ�
�ʴ�Ϊ��NaOH����Һ�����ȣ�Ũ���Ტ���ȣ�
��4��A���ڱ���ͬϵ�B����±������������A����B�ķ�Ӧ������ȡ����Ӧ��D�к���̼̼˫���������D����G�ķ�Ӧ�����Ǽӳɷ�Ӧ���ʴ�Ϊ��ȡ����Ӧ���ӳɷ�Ӧ��
��5����ΪC��E���Է���������Ӧ������E�����ᣬ����ΪC��C6H5CHOHCH3�������������Ľṹ��ʽ��
 ��
���ʴ�Ϊ��
 ��
����6��������һ�����IJ���ֻ��һ�֣�˵��Ӧ���ǶԳ��Խṹ������G�ķ���ʽC8H8Br2��֪���������Ĺ�������7�֣�

���к˴Ź�������������壬�ҷ������Ϊl��1����
 ��
���ʴ�Ϊ��7��
 ��
��������������Ҫ�����л���ṹʽ��ȷ�����л���������ƶϡ�ͬ���칹�����д���жϡ��л���Ӧ����ʽ����д�ͷ�Ӧ���͵��жϣ���Ŀ�Ѷ��еȣ��״���Ϊͬ���칹����жϣ�ע����ȷ�ƶ��л���ĽṹΪ������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ