��Ŀ����
(15��)���ſ�ѧ�����ķ�չ���ⶨ�����ӵ��������ֶ�Խ��Խ�࣬�ⶨ�ľ�ȷ��ҲԽ��Խ�ߡ�����һ�ּ��еIJⶨ���������������������£����÷�����ƽ��ȡ��ϸ�����NaCl����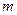 g�����ΪV1�����Ķ�������A�У����õζ�����������A�м��뱽�����������ӱ�����������A�Ŀ̶���ʱ�����뱽�����ΪV2�������������ϲ����ش��������⣺
g�����ΪV1�����Ķ�������A�У����õζ�����������A�м��뱽�����������ӱ�����������A�Ŀ̶���ʱ�����뱽�����ΪV2�������������ϲ����ش��������⣺

��1����������A�����________________(��ʵ����������)��
��2���ζ���������ʽ�ζ��ܻ����ü�ʽ�ζ��ܣ�_____________________��Ϊʲô��
______________________________________________________________��
��3���ܷ���ˮ���汽��_____________��Ϊʲô��
_______________________________________________________��
��4���ⶨ���ӻ����������Ӽ�ļ���ķ�������X���������䷨����֪X�����������Dz����NaCl����������Na����Cl���ĺ˼��Ϊ cm������������������õİ����ӵ���������ѧ����ʽΪ��
cm������������������õİ����ӵ���������ѧ����ʽΪ��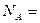 ______________________��
______________________��
��5�����ײ��ϵı���ԭ��ռ��ԭ�����ı����������������������������ʵ�ԭ����ij�������Ĵ�С����״ǡ�õ����Ȼ��ƾ����Ĵ�С����״���������������ı���ԭ��ռ��ԭ�����İٷֱ�Ϊ��______________��
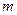 g�����ΪV1�����Ķ�������A�У����õζ�����������A�м��뱽�����������ӱ�����������A�Ŀ̶���ʱ�����뱽�����ΪV2�������������ϲ����ش��������⣺
g�����ΪV1�����Ķ�������A�У����õζ�����������A�м��뱽�����������ӱ�����������A�Ŀ̶���ʱ�����뱽�����ΪV2�������������ϲ����ش��������⣺
��1����������A�����________________(��ʵ����������)��
��2���ζ���������ʽ�ζ��ܻ����ü�ʽ�ζ��ܣ�_____________________��Ϊʲô��
______________________________________________________________��
��3���ܷ���ˮ���汽��_____________��Ϊʲô��
_______________________________________________________��
��4���ⶨ���ӻ����������Ӽ�ļ���ķ�������X���������䷨����֪X�����������Dz����NaCl����������Na����Cl���ĺ˼��Ϊ
 cm������������������õİ����ӵ���������ѧ����ʽΪ��
cm������������������õİ����ӵ���������ѧ����ʽΪ��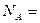 ______________________��
______________________����5�����ײ��ϵı���ԭ��ռ��ԭ�����ı����������������������������ʵ�ԭ����ij�������Ĵ�С����״ǡ�õ����Ȼ��ƾ����Ĵ�С����״���������������ı���ԭ��ռ��ԭ�����İٷֱ�Ϊ��______________��
��1������ƿ����2�֣���2������ʽ�ζ��ܣ���1�֣����ܸ�ʴ��ʽ�ζ��ܵ���Ƥ���ܽ⣩����2�֣�
��3�����ܣ���1�֣���NaCl����ˮ�������NaCl���������2�֣�
��4��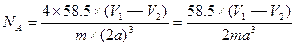 ��4�֣�
��4�֣�
NaCl���ܶ�Ϊ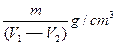 ��NaCl���������Ϊ
��NaCl���������Ϊ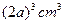 ����NaCl����������Ϊ
����NaCl����������Ϊ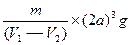 ��һ��NaCl������4����NaCl������ÿ����NaCl��������Ϊ
��һ��NaCl������4����NaCl������ÿ����NaCl��������Ϊ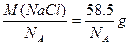 ���ʣ�
���ʣ� ��
��
�� ��
��
��5����NaCl�ľ���ͼ��֪��NaCl�ľ���Ϊ��������ṹ�������������ֻ��һ��Na+�������������Ӷ���������������ϣ������е���ԭ����Ϊ27�����������Ͼ���26�����ʡ�NaCl���������ı���ԭ��ռ��ԭ�����İٷֱ�Ϊ ����3�֣�
����3�֣�
��3�����ܣ���1�֣���NaCl����ˮ�������NaCl���������2�֣�
��4��
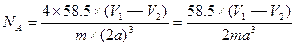 ��4�֣�
��4�֣�NaCl���ܶ�Ϊ
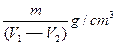 ��NaCl���������Ϊ
��NaCl���������Ϊ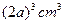 ����NaCl����������Ϊ
����NaCl����������Ϊ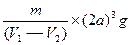 ��һ��NaCl������4����NaCl������ÿ����NaCl��������Ϊ
��һ��NaCl������4����NaCl������ÿ����NaCl��������Ϊ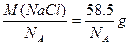 ���ʣ�
���ʣ� ��
����
 ��
����5����NaCl�ľ���ͼ��֪��NaCl�ľ���Ϊ��������ṹ�������������ֻ��һ��Na+�������������Ӷ���������������ϣ������е���ԭ����Ϊ27�����������Ͼ���26�����ʡ�NaCl���������ı���ԭ��ռ��ԭ�����İٷֱ�Ϊ
 ����3�֣�
����3�֣���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ