��Ŀ����
����Ŀ�������仯�����ڿ�ѧ�о��ͻ����������������Ź㷺��Ӧ�á���ش��������⣺
��1���뵪Ԫ��ͬ��ĵ�������Ԫ�صĻ�̬ԭ�Ӽ۲���ӹ������ʽΪ___________��
��2�����ط��ӵĽṹ��ʽΪ��CO(NH2)2���÷�����������ĿΪ___________��ʵ���ã������е�����ԭ����ͬһƽ���ڣ������е�̼������125pm���ȵ��͵�̼��˫����121pm�������������е�̼������137pm���ȵ��͵�̼��������147pm���̣�˵�������д���____________��
��3�������ӹ���Ϊ_________���������У��������е�ÿ��H ������һ��������γɣ�1 mol��̬������_____mol�����
��4��ͨ����ΪCu3N�����Ӿ��壬�侧���ܿ�ͨ��ͼ(a)��Born-Haberѭ������õ���

��֪�� Cuԭ�ӵĵ�һ������Ϊ_____kJmol1��N��N������Ϊ_____kJmol1��Cu3N������Ϊ_____kJmol1��
��5��Cu3N����ľ�����ͼ(b)��ʾ�������߳�Ϊanm��������N3- λ��Cu+���γɵ����� ��������ģ�����������ı߳�Ϊ____nm��
���𰸡�![]() 7 ��46 ������ 3 756 936 5643
7 ��46 ������ 3 756 936 5643 ![]() a
a
��������
��1���뵪Ԫ��ͬ��ĵ�������Ԫ��ΪAs����ԭ�Ӽ۲���ӹ��ʽΪ![]() ��
��
��2�����صĽṹ��ʽΪ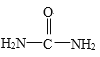 ���ɼ�ԭ��֮��ֻ���γ�һ���Ҽ�����˸����ط����к��ЦҼ�����ĿΪ7��ʵ���ã������е�����ԭ����ͬһƽ���ڣ������е�̼������125pm���ȵ��͵�̼��˫����121pm�������������е�̼������137pm���ȵ��͵�̼��������147pm���̣������뱽��̼̼������̼̼������̼̼˫��֮��ļ���˵�����ط����е�ԭ�ӵŵ��Ӷ���C=O�еĦм��γ���һ��4����6���ӵ������м����������к��Ц�46��
���ɼ�ԭ��֮��ֻ���γ�һ���Ҽ�����˸����ط����к��ЦҼ�����ĿΪ7��ʵ���ã������е�����ԭ����ͬһƽ���ڣ������е�̼������125pm���ȵ��͵�̼��˫����121pm�������������е�̼������137pm���ȵ��͵�̼��������147pm���̣������뱽��̼̼������̼̼������̼̼˫��֮��ļ���˵�����ط����е�ԭ�ӵŵ��Ӷ���C=O�еĦм��γ���һ��4����6���ӵ������м����������к��Ц�46��
��3��NH3�ռ乹��Ϊ�����Σ����������е�ÿ��H������һ��������γɡ���֪��ÿһ��Hԭ��ֱ��������Nԭ���ڽӣ����ݾ���˼�룬ÿһ��Hԭ�ӽ��������Χ��ÿһ��Nԭ�ӹ���1/2��Hԭ�ӣ������廯ѧʽΪNH3�İ������У�ÿ��Nԭ�Ӿ��ֺ�Ӧ������Hԭ���������ڣ�����ھ���ǰ�ľ���ṹ�У�ÿ��Nԭ��Ӧ������Hԭ���ڽӣ�ÿ��Hԭ�Ӳ���һ��������γɣ���Ȼ��ÿ�����ֻ��һ��Hԭ�Ӳ����γɣ����ݾ���˼�룬ÿ�������Ӿ��ֵõ�����Hԭ�ӣ�Ҳ��ÿ�������Ӿ��ֵõ�3���������1mol������3mol�����
��4�����ݵ�һ�����ܵĶ��壬Cuԭ�ӵĵ�һ������Ϊ2268/3kJ��mol��1=756kJ��mol��1���������γ�1mol��ѧ��ʱ��Ҫ�ų���͵������������������ļ���Ϊ468kJ��mol��1��2=936kJ��mol��1���赪����ͭ�ľ�����ΪU�����ݸ�˹���ɿ�֪��1017+2268+468-1290-U=-3180��ע��ͭԭ�ӱ�Ϊ��ͭ���������ȵģ�����ԭ�ӱ�Ϊ�������Ƿ��ȵģ�����U=5643kJ��mol-1,���ԣ�Cu3N�ľ�����Ϊ5643kJ��mol��1��
��5��������N3�� λ��Cu�����γɵ�������������ģ�������Cu��������ΪN3��������������ı߳�Ϊ��Խ���Ϊһ�룬��Ϊ![]() anm��
anm��






