��Ŀ����
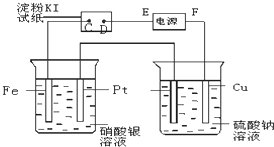 ��ͼ��ʾ��װ�ý��е�⣮ͨ��һ��ʱ�����ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ��
��ͼ��ʾ��װ�ý��е�⣮ͨ��һ��ʱ�����ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ����1����ԴEΪ
��
��
������2��D�缫�ĵ缫��ӦʽΪ
2H++2e=H2��
2H++2e=H2��
����3������ձ��з�����Ӧ�Ļ�ѧ����ʽΪ��
4AgNO3+2H2O
4Ag+O2��+4HNO3
| ||
4AgNO3+2H2O
4Ag+O2��+4HNO3
��
| ||
��4�����ұ��й۲쵽��������
ͭƬ�ܽ⣬�������ɡ���ɫ����
ͭƬ�ܽ⣬�������ɡ���ɫ����
����5�������£��ӵ�ʼ��ʱ��t s��A��Bװ���й��ռ�������0.168L��STP������������������������Ӧ���������ⶨ����A����Һ��ˮϡ��Ϊ1000mL����A��Һ��H+��Ũ��Ϊ
0.01mol/L
0.01mol/L
��������ͨ��һ��ʱ�����ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ����C�˷���2I--2e-=I2����C��Ϊ������DΪ��������EΪ��Դ�ĸ�����FΪ��Դ��������������ӵķŵ�˳�����ĵ缫��Ӧ��������
����⣺ͨ��һ��ʱ�����ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ����C�˷���2I--2e-=I2����C��Ϊ������DΪ��������EΪ��Դ�ĸ�����FΪ��Դ��������
��1��EΪ��Դ�ĸ������ʴ�Ϊ������
��2����D�缫Ϊ���������������������ŵ磬�缫��ӦΪ2H++2e=H2�����ʴ�Ϊ��2H++2e=H2����
��3������ձ��з��������������Һ�ķ�Ӧ���õ�ⷴӦΪ4AgNO3+2H2O
4Ag+O2��+4HNO3��
�ʴ�Ϊ��4AgNO3+2H2O
4Ag+O2��+4HNO3��
��4���Ҳ��ձ��з�����������Cu-2e-=Cu2+����������2H2O+2e=H2��+2OH-����۲쵽ͭƬ�ܽ⣬�������ɡ���ɫ�������ʴ�Ϊ��ͭƬ�ܽ⣬�������ɡ���ɫ������
��5����װ����O2����4e-��2H2����A��Bװ���й��ռ�������0.168L��STP������������ʵ���Ϊ
=0.0075mol����n��O2��=0.0025mol��
��4AgNO3+2H2O
4Ag+O2��+4HNO3��֪��n��H+��=0.0025mol��4=0.01mol������c��H+��=
=0.01mol/L���ʴ�Ϊ��0.01mol/L��
��1��EΪ��Դ�ĸ������ʴ�Ϊ������
��2����D�缫Ϊ���������������������ŵ磬�缫��ӦΪ2H++2e=H2�����ʴ�Ϊ��2H++2e=H2����
��3������ձ��з��������������Һ�ķ�Ӧ���õ�ⷴӦΪ4AgNO3+2H2O
| ||
�ʴ�Ϊ��4AgNO3+2H2O
| ||
��4���Ҳ��ձ��з�����������Cu-2e-=Cu2+����������2H2O+2e=H2��+2OH-����۲쵽ͭƬ�ܽ⣬�������ɡ���ɫ�������ʴ�Ϊ��ͭƬ�ܽ⣬�������ɡ���ɫ������
��5����װ����O2����4e-��2H2����A��Bװ���й��ռ�������0.168L��STP������������ʵ���Ϊ
| 0.168L |
| 22.4L/mol |
��4AgNO3+2H2O
| ||
| 0.01mol |
| 1L |
���������⿼��ԭ��غ͵��ص�ԭ����ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫΪ������ͻ�ƿڣ���Ϥ���ӵķŵ�˳�����ĵ缫��Ӧ���ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ


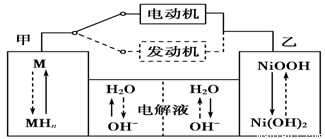
 2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����
2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����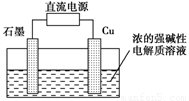
 Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��
Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��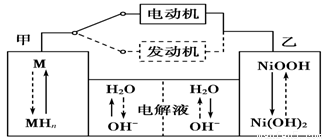
 2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����
2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����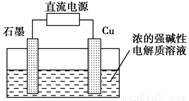
 Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��
Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��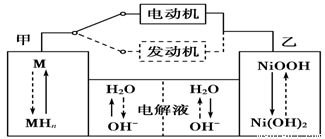
 2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����
2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����
 Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��
Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��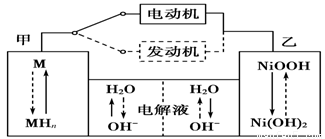
 2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����
2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����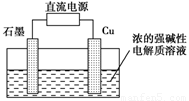
 Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��
Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��