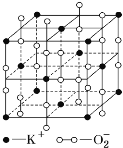
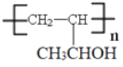ΧβΡΩΡΎ»ί
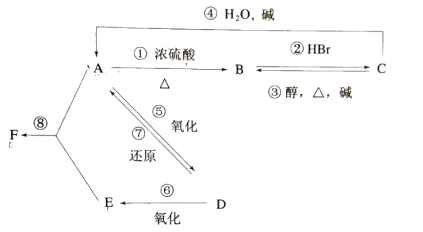
ΓΨΧβΡΩΓΩΡ≥”–ΜζΈο AΘ§”… CΓΔHΓΔO»ΐ÷÷‘ΣΥΊΉι≥…Θ§‘Ύ“ΜΕ®ΧθΦΰœ¬Θ§”… AΩ…“‘ΉΣΜ·ΈΣ”–ΜζΈο BΓΔC ΚΆ DΓΔEΘΜC”÷Ω…“‘ΉΣΜ·ΈΣ BΓΔAΓΘΥϋΟ«ΒΡΉΣΜ·ΙΊœΒ»γœ¬ΘΚ
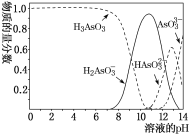
“―÷Σ D ΒΡ’τΤχΟήΕ» ««βΤχΒΡ 22 ±ΕΘ§≤ΔΩ…“‘ΖΔ…ζ“χΨΒΖ¥”ΠΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©D ΒΡΖ÷Ή” Ϋ «______________________
Θ®2Θ©F ΒΡΟϊ≥Τ «_________________________
Θ®3Θ©‘ΎΔΌΔΎΔέΔήΒ»Ζ¥”Π÷–Θ§ τ”Ύœϊ»ΞΖ¥”ΠΒΡ «________________________ΓΘ
Θ®4Θ©‘Ύ AΓΔBΓΔCΓΔDΓΔEΓΔF ÷–Θ§Ρή”κΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΖ¥”ΠΒΡ «Θ®ΧνΉ÷ΡΗΘ©________________________
ΓΨ¥πΑΗΓΩCH3CHO ““Υα““θΞ ΔΌΔέ ABD
ΓΨΫβΈωΓΩ
DΒΡ’τΤχΟήΕ» ««βΤχΒΡ22±ΕΘ§‘ρœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ44Θ§≤ΔΩ…“‘ΖΔ…ζ“χΨΒΖ¥”ΠΘ§ΥΒΟςΚ§”–-CHOΘ§‘ρDΈΣCH3CHOΘ§”…ΉΣΜ·ΙΊœΒΩ…÷ΣEΈΣCH3COOHΘ§AΈΣCH3CH2OHΘ§BΈΣCH2=CH2Θ§CΈΣCH3CH2BrΘ§‘ρFΈΣCH3COOC2H5Θ§ΫαΚœ”–ΜζΈοΒΡ–‘÷ “‘ΦΑΙΌΡήΆ≈ΒΡ±δΜ·Ϋβ¥πΗΟΧβΓΘ
Θ®1Θ©”……œ ωΖ÷ΈωΩ…÷ΣΘ§DΈΣCH3CHOΘΜ
Θ®2Θ©FΈΣCH3COOC2H5Θ§ΤδΟϊ≥ΤΈΣ““Υα““θΞΘΜ
Θ®3Θ©ΔΌΈΣCH3CH2OH‘Ύ≈®ΝρΥαΦ”»»ΧθΦΰœ¬Ζ¥”Π…ζ≥…CH2=CH2Θ§Ζ¥”ΠΈΣ¥ΦΒΡœϊ»ΞΖ¥”ΠΘΜ
ΔΎΈΣCH2=CH2”κHBrΖ¥”Π…ζ≥…CH3CH2BrΘ§ τ”Ύœ©ΧΰΒΡΦ”≥…Ζ¥”ΠΘΜ
ΔέΈΣCH3CH2Br‘ΎΦνΓΔ¥ΦΓΔΦ”»»ΧθΦΰœ¬Ζ¥”Π…ζ≥…CH2=CH2Θ§ τ”Ύ¬±¥ζΧΰΒΡœϊ»ΞΖ¥”ΠΘΜ
ΔήΈΣCH3CH2Br‘ΎΦνΒΡΥ°»ή“Κ÷–Ζ¥”Π…ζ≥…CH3CH2OHΘ§ τ”Ύ¬±¥ζΧΰΒΡΥ°Ϋβ(Μρ»Γ¥ζ)Ζ¥”ΠΘΜ
Ι τ”Ύœϊ»ΞΖ¥”ΠΒΡ «ΘΚΔΌΔέΘΜ
Θ®4Θ©AΈΣCH3CH2OHΘ§BΈΣCH2=CH2Θ§CΈΣCH3CH2BrΘ§DΈΣCH3CHOΘ§EΈΣCH3COOHΘ§FΈΣCH3COOC2H5Θ§A÷–Κ§”–τ«ΜυΓΔB÷–Κ§”–ΧΦΧΦΥΪΦϋΓΔD÷–Κ§”–»©ΜυΘ§ΨυΡή ΙΥα–‘ΗΏΟΧΥαΦΊΖ¥”ΠΘ§Ι ¥πΑΗΈΣΘΚABDΓΘ

ΓΨΧβΡΩΓΩ”Ο»γΆΦΥυ ΨΉΑ÷ΟΦλ―ι““œ© ±≤Μ–η“Σ≥ΐ‘”ΒΡ «Θ® Θ©
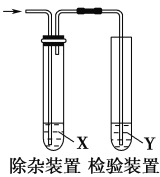
―Γœν | ““œ©ΒΡ÷Τ±Η | ‘ΦΝX | ‘ΦΝY |
A | CH3CH2Br”κNaOH““¥Φ»ή“ΚΙ≤»» | H2O | KMnO4Υα–‘»ή“Κ |
B | CH3CH2Br”κNaOH““¥Φ»ή“ΚΙ≤»» | H2O | Br2ΒΡCCl4»ή“Κ |
C | C2H5OH”κ≈®H2SO4Φ”»»÷Ν170Γφ | NaOH»ή“Κ | KMnO4Υα–‘»ή“Κ |
D | C2H5OH”κ≈®H2SO4Φ”»»÷Ν170Γφ | NaOH»ή“Κ | δεΥ° |
A.AB.BC.CD.D
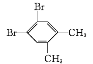
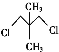
ΓΨΧβΡΩΓΩΧΦΓΔΝΉΓΔΝρΒ»‘ΣΥΊ–Έ≥…ΒΡΒΞ÷ ΚΆΜ·ΚœΈο‘Ύ…ζΜνΓΔ…ζ≤ζ÷–”–÷Ί“ΣΒΡ”ΟΆΨΓΘ
(1)œ¬Ν–ΒΣ‘≠Ή”ΒΡΒγΉ”≈≈≤ΦΆΦ±μ ΨΒΡΉ¥Χ§÷–Θ§ΡήΝΩ”…ΒΆΒΫΗΏΒΡΥ≥–ρ «____(ΧνΉ÷ΡΗ)ΓΘ
A. ![]()
B. ![]()
C. ![]()
D. ![]()
(2)P4S3Ω…”Ο”Ύ÷Τ‘λΜπ≤ώΘ§ΤδΖ÷Ή”ΫαΙΙ»γΆΦΥυ ΨΘΚ

ΔΌP4S3Ζ÷Ή”÷–Νρ‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–ΆΈΣ____ΓΘ
ΔΎΟΩΗωP4S3Ζ÷Ή”÷–Κ§”–ΒΡΙ¬ΒγΉ”Ε‘ΒΡ ΐΡΩΈΣ____Ε‘ΓΘ
(3)ΩΤ―ßΦ“Κœ≥…ΝΥ“Μ÷÷―τάκΉ”ΓΑN5n+Γ±Θ§ΤδΫαΙΙ «Ε‘≥ΤΒΡΘ§5ΗωN≈≈≥…ΓΑVΓ±–ΈΘ§ΟΩΗωNΕΦ¥οΒΫ8ΒγΉ”Έ»Ε®ΫαΙΙΘ§«“Κ§”–2ΗωΒΣΒΣ»ΐΦϋΘ§¥ΥΚσ”÷Κœ≥…ΝΥ“Μ÷÷Κ§”–ΓΑN5n+Γ±ΒΡΜ·―ß ΫΈΣΓΑN8Γ±ΒΡάκΉ”ΨßΧε(ΗΟΨßΧε÷–ΟΩΗωN‘≠Ή”ΕΦ¥οΒΫΝΥ8ΒγΉ”Έ»Ε®ΫαΙΙ)Θ§N8ΒΡΒγΉ” ΫΈΣ____ΓΘ(CN)2÷–Φϋ”κΦϋ÷°ΦδΒΡΦ–Ϋ«ΈΣ180ΓψΘ§≤Δ”–Ε‘≥Τ–‘Θ§Ζ÷Ή”÷–ΟΩΗω‘≠Ή”ΒΡΉνΆβ≤ψΨυ¬ζΉψ8ΒγΉ”Έ»Ε®ΫαΙΙΘ§ΤδΫαΙΙ ΫΈΣ____ΓΘ
(4)÷±Ν¥ΕύΝΉΥαΗυ“θάκΉ” «”…ΝΫΗωΜρΝΫΗω“‘…œΝΉ―θΥΡΟφΧεΆ®ΙΐΙ≤”ΟΕΞΫ«―θ‘≠Ή”Ν§Ϋ”Τπά¥ΒΡΘ§ΤδΫαΙΙ»γΆΦΥυ ΨΓΘ‘ρ”…nΗωΝΉ―θΥΡΟφΧε–Έ≥…ΒΡ’βάύΝΉΥαΗυάκΉ”ΒΡΆ® ΫΈΣ____ΓΘ

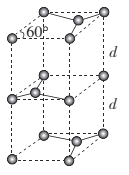
(5)ΧΦΥα―Έ÷–ΒΡ―τάκΉ”≤ΜΆ§Θ§»»Ζ÷ΫβΈ¬Ε»ΨΆ≤ΜΆ§ΓΘœ¬±μΈΣΥΡ÷÷ΧΦΥα―ΈΒΡ»»Ζ÷ΫβΈ¬Ε»ΚΆΕ‘”ΠΫπ τ―τάκΉ”ΒΡΑκΨΕΓΘΥφΉ≈Ϋπ τ―τάκΉ”ΑκΨΕΒΡ‘ω¥σΘ§ΧΦΥα―ΈΒΡ»»Ζ÷ΫβΈ¬Ε»÷πΫΞ…ΐΗΏΘ§‘≠“ρ « ___ΓΘ
ΧΦΥα―Έ | MgCO3 | CaCO3 | SrCO3 | BaCO3 |
»»Ζ÷ΫβΈ¬Ε»/Γφ | 402 | 900 | 1172 | 1360 |
Ϋπ τ―τάκΉ”ΑκΨΕ/pm | 66 | 99 | 112 | 135 |
(6) ·ΡΪΒΡΨßΑϊΫαΙΙ»γΆΦΥυ ΨΓΘ“―÷Σ ·ΡΪΒΡΟήΕ»ΈΣΠ―g.cm-3Θ§C-CΦϋΒΡΦϋ≥ΛΈΣr cmΘ§MΈΣΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷ΒΘ§‘ρ ·ΡΪΨßΧεΒΡ≤ψΦδΨύd= ___cmΓΘ