��Ŀ����
(19��)
������CH4��C2H4��C2H2��C2H6��C3H8�����л���ش��������⣺
��������ͬʱ:����ͬ״�������������__ ____����ȫȼ��ʱ���ģ�2����������__ __������CO2������___ _������ˮ������___ ___��
��ͬ��ͬѹͬ���ʱ������������ȫȼ������O2����������__
��:�л���ѧ�еķ�Ӧ���ͽ϶࣬�����з�Ӧ���ࣨ����ţ���
���ұ����Ʊ���
�������ڿ�����ȼ�բ���ϩʹ������Ȼ�̼��Һ��ɫ����ϩʹ���Ը��������Һ��ɫ������ϩ�ƾ���ϩ�����������ڹ��յ������·�Ӧ����������������Ƶ��Ҵ���Һ���Ȣ��ɼױ���ȡTNT
��1����������ȡ����Ӧ���� ��
����������Ӧ���� �����ڼӳɷ�Ӧ���� ��
����ȥ��Ӧ���� ���ھۺϷ�Ӧ����
��2��д���ߺ͢�Ļ�ѧ����ʽ
��
��
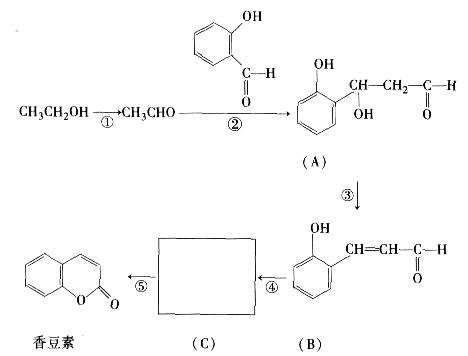
��ҽҩ��˹ƥ�ֵĽṹ��ʽ��ͼ1��ʾ��


ͼ1 ͼ2
�Ը��ݰ�˹ƥ�ֵĽṹ�ش�
�Ű�˹ƥ�ֿ����������ʣ��ڷ�����θ��ø�������£���˹ƥ�ַ���ˮ�ⷴӦ������A��B �������ʡ�����A�Ľṹ��ʽ��ͼ2��ʾ����B�Ľṹ��ʽΪ ��B�к��еĹ����������� ��
�ư�˹ƥ�ָ�NaHCO3ͬʱ���ã���ʹ����ˮ�����A��NaHCO3��Ӧ�����ɿ�����������Һ�ų����˿������εĽṹ��ʽ��
��д����˹ƥ������������������Һ���ȵĻ�ѧ��Ӧ����ʽ
������CH4��C2H4��C2H2��C2H6��C3H8�����л���ش��������⣺
��������ͬʱ:����ͬ״�������������__ ____����ȫȼ��ʱ���ģ�2����������__ __������CO2������___ _������ˮ������___ ___��
��ͬ��ͬѹͬ���ʱ������������ȫȼ������O2����������__
��:�л���ѧ�еķ�Ӧ���ͽ϶࣬�����з�Ӧ���ࣨ����ţ���
���ұ����Ʊ���

�������ڿ�����ȼ�բ���ϩʹ������Ȼ�̼��Һ��ɫ����ϩʹ���Ը��������Һ��ɫ������ϩ�ƾ���ϩ�����������ڹ��յ������·�Ӧ����������������Ƶ��Ҵ���Һ���Ȣ��ɼױ���ȡTNT
��1����������ȡ����Ӧ���� ��
����������Ӧ���� �����ڼӳɷ�Ӧ���� ��
����ȥ��Ӧ���� ���ھۺϷ�Ӧ����
��2��д���ߺ͢�Ļ�ѧ����ʽ
��
��
��ҽҩ��˹ƥ�ֵĽṹ��ʽ��ͼ1��ʾ��


ͼ1 ͼ2
�Ը��ݰ�˹ƥ�ֵĽṹ�ش�
�Ű�˹ƥ�ֿ����������ʣ��ڷ�����θ��ø�������£���˹ƥ�ַ���ˮ�ⷴӦ������A��B �������ʡ�����A�Ľṹ��ʽ��ͼ2��ʾ����B�Ľṹ��ʽΪ ��B�к��еĹ����������� ��
�ư�˹ƥ�ָ�NaHCO3ͬʱ���ã���ʹ����ˮ�����A��NaHCO3��Ӧ�����ɿ�����������Һ�ų����˿������εĽṹ��ʽ��
��д����˹ƥ������������������Һ���ȵĻ�ѧ��Ӧ����ʽ
��:(1)�ã�4���ã�4����2��2�����ã�4���ƣ�3��8
��:�ޢࣻ�ڢܣ��٢ۣ��ߣ��ݣ��������÷֣���ȫ�ߵ�һ��֣�
��2���ߣ�
��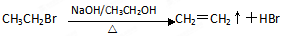
�ࣺ
��1��CH3COOH���Ȼ� ��2��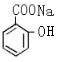
�� +3NaOH ��
+3NaOH ��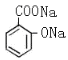 + CH3COONa +2H2O
+ CH3COONa +2H2O
��:�ޢࣻ�ڢܣ��٢ۣ��ߣ��ݣ��������÷֣���ȫ�ߵ�һ��֣�
��2���ߣ�

��
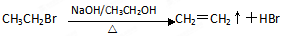
�ࣺ

��1��CH3COOH���Ȼ� ��2��

��
 +3NaOH ��
+3NaOH �� + CH3COONa +2H2O
+ CH3COONa +2H2O��1������ͬ�����£���������ʵ���Խ�����Խ�ࡣ��������ͬʱ��Ħ������ԽС�����ʵ���Խ�࣬��������ͬ״������������Ǽ��顣�������к�����Խ�ߣ����ĵ�����Խ�࣬���Ի��Ǽ��顣��̼��Խ�ߣ����ɵ�CO2Խ�࣬����Ӧ������Ȳ���������ߣ�����ˮ��Խ�࣬����Ǽ��顣
��2����������ȼ��ͨʽCnHm����n��m/4��O2��nCO2��m/2H2O��֪�������ʵ�����ͬʱ�����������Ķ�����n��m/4�����ȣ����Ը��ݷ���ʽ��֪���������ĵ�������ࡣ
��:��1���л�����ӵ�ijЩԭ�ӻ�ԭ���ű�����ԭ�ӻ�ԭ���������������������ʵķ�Ӧ��ȡ����Ӧ�����Դ�ѡ�ޢࣻ������ȥ��ķ�Һʱ������Ӧ����˴�ѡ�ڢܣ��л��������˫�����������˵�̼ԭ��������ԭ�ӻ�ԭ����ֱ�ӽ�������µĻ�����ķ�Ӧ�мӳɷ�Ӧ������ȷ�Ĵ�ѡ�٢ۣ��л���������һ��������,��һ����������ȥһ����С����(��ˮ��±����ȷ���)�������ɲ����ͣ�̼̼˫����������������ķ�Ӧ��������ȥ��Ӧ�����Ǣߡ��л�С�������ɸ߷��ӻ�����ķ�Һ�ǾۺϷ�Ӧ�����ѡ�ݡ�
��1����˾ƥ���к����������ܷ���ˮ�ⷴӦ�����ݰ�˾ƥ�ֺ�A�Ľṹ��ʽ��֪��B�Ľṹ��ʽΪCH3COOH����Ϊ���ᣬ�����Ȼ���
��2��A�к����Ȼ��ͷ��ǻ������з��ǻ���̼�����Ʋ���Ӧ������������Ľṹ��ʽΪ ��
��
��3����˾ƥ���к���1���Ȼ���1����������ˮ���������1�����ǻ��������ܺ�3molȥ�������Ʒ�Ӧ��
��2����������ȼ��ͨʽCnHm����n��m/4��O2��nCO2��m/2H2O��֪�������ʵ�����ͬʱ�����������Ķ�����n��m/4�����ȣ����Ը��ݷ���ʽ��֪���������ĵ�������ࡣ
��:��1���л�����ӵ�ijЩԭ�ӻ�ԭ���ű�����ԭ�ӻ�ԭ���������������������ʵķ�Ӧ��ȡ����Ӧ�����Դ�ѡ�ޢࣻ������ȥ��ķ�Һʱ������Ӧ����˴�ѡ�ڢܣ��л��������˫�����������˵�̼ԭ��������ԭ�ӻ�ԭ����ֱ�ӽ�������µĻ�����ķ�Ӧ�мӳɷ�Ӧ������ȷ�Ĵ�ѡ�٢ۣ��л���������һ��������,��һ����������ȥһ����С����(��ˮ��±����ȷ���)�������ɲ����ͣ�̼̼˫����������������ķ�Ӧ��������ȥ��Ӧ�����Ǣߡ��л�С�������ɸ߷��ӻ�����ķ�Һ�ǾۺϷ�Ӧ�����ѡ�ݡ�
��1����˾ƥ���к����������ܷ���ˮ�ⷴӦ�����ݰ�˾ƥ�ֺ�A�Ľṹ��ʽ��֪��B�Ľṹ��ʽΪCH3COOH����Ϊ���ᣬ�����Ȼ���
��2��A�к����Ȼ��ͷ��ǻ������з��ǻ���̼�����Ʋ���Ӧ������������Ľṹ��ʽΪ
 ��
����3����˾ƥ���к���1���Ȼ���1����������ˮ���������1�����ǻ��������ܺ�3molȥ�������Ʒ�Ӧ��

��ϰ��ϵ�д�
�����Ŀ
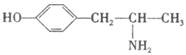
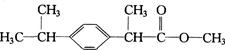 �����л���X��˵���У��������
�����л���X��˵���У�������� 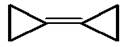
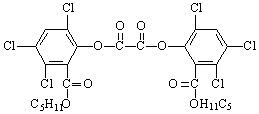
 ��ʾ�ķ���ʽ �������� ��
��ʾ�ķ���ʽ �������� ��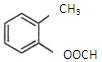 �к��еĹ����ŵ�����Ϊ �����京����ͬ�����ţ��ҷ��ӽṹ�б�����ֻ��һ��ȡ������ͬ���칹���� �֡�д�����ǵĽṹ��ʽ ��
�к��еĹ����ŵ�����Ϊ �����京����ͬ�����ţ��ҷ��ӽṹ�б�����ֻ��һ��ȡ������ͬ���칹���� �֡�д�����ǵĽṹ��ʽ ��