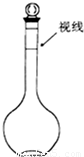
��Ŀ����
����400mL0.1mol/L��Na2CO3��Һ��
��1�����㣺����ˮNa2CO3
��3���ܽ⣺��������
��4��ת�ƣ����ձ��е���Һ��
��5�����ݣ���������ƿ�������ˮʱ����̶���
���࣬����
��6�������������������һ�����ʵ���Ũ�ȵ���Һ��������Ϊ
��7����Һע������ƿǰ��ָ������£�������Ϊ
��8���ý�ͷ�ιܶ��ݺ���������Һ����ڿ̶���ʱ��Ҫ�ټ�ˮ��������Ϊ
��9�������ˮ����ʱ�����˿̶��ߣ����ܽ��������������ߣ���
��10�����ҡ��ʱ��С���������Σ������ټ�ˮ���̶ȣ������������ƣ�������Ϊ
��11�������ܽ��ת��������ƿʱ����������������ˮ���ձ���������ϴ��2-3�Σ�����ϴ��Һһ����������ƿ��������Ϊ
��1�����㣺����ˮNa2CO3
5.3
5.3
g�� ��2������������ƽ������ˮNa2CO35.3
5.3
g����3���ܽ⣺��������
�ձ���������
�ձ���������
����4��ת�ƣ����ձ��е���Һ��
������
������
С�ĵ�����������ƿ
����ƿ
�У���5�����ݣ���������ƿ�������ˮʱ����̶���
1-2
1-2
cm��ֹͣ��Ϊ�����ˮ��������࣬����
��ͷ�ι�
��ͷ�ι�
������ˮ����Һ����Һ��
��Һ��
������̶�������
����
����������������ݣ���6�������������������һ�����ʵ���Ũ�ȵ���Һ��������Ϊ
����ƿ���ݻ��̶�
����ƿ���ݻ��̶�
����7����Һע������ƿǰ��ָ������£�������Ϊ
��Һ���������������ʣ��������ȴ�����£��ᵼ����Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
��Һ���������������ʣ��������ȴ�����£��ᵼ����Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
����8���ý�ͷ�ιܶ��ݺ���������Һ����ڿ̶���ʱ��Ҫ�ټ�ˮ��������Ϊ
�ټ�ˮ������Һ�����ƫ��������Һ��Ũ��ƫС��
�ټ�ˮ������Һ�����ƫ��������Һ��Ũ��ƫС��
����9�������ˮ����ʱ�����˿̶��ߣ����ܽ��������������ߣ���
��������
��������
����10�����ҡ��ʱ��С���������Σ������ټ�ˮ���̶ȣ������������ƣ�������Ϊ
�ټ�ˮ���̶ȵ������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
�ټ�ˮ���̶ȵ������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
����11�������ܽ��ת��������ƿʱ����������������ˮ���ձ���������ϴ��2-3�Σ�����ϴ��Һһ����������ƿ��������Ϊ
�������ϴ��Һ��������ƿ�ᵼ�����ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
�������ϴ��Һ��������ƿ�ᵼ�����ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
����������1����2������m=nM=CVM����̼���Ƶ�������
��3����4����5�����ݲ�������ѡ��ʵ��������
��6����������ƿ���ص������
��7������̼�����ܽ���ص������
��8����9����10����11�������Ƿ�Ӱ��������Һ��Ũ�ȷ�����
��3����4����5�����ݲ�������ѡ��ʵ��������
��6����������ƿ���ص������
��7������̼�����ܽ���ص������
��8����9����10����11�������Ƿ�Ӱ��������Һ��Ũ�ȷ�����
����⣺��1��m=nM=CVM=0.1mol/L��0.5L��106g/mol=5.3g��
�ʴ�Ϊ��5.3��
��2����������̼����5.3g��
�ʴ�Ϊ��5.3��
��3���ձ�ʢ�Ź���̼���ƣ���������������ܽ⣮
�ʴ�Ϊ���ձ�����������
��4��ת�ƣ����ձ��е���Һ�ز�����С�ĵ������� ����ƿ�У�
�ʴ�Ϊ��������������ƿ��
��5�����ݣ���������ƿ�������ˮʱ����̶��� 1-2cm��ֹͣ��Ϊ�����ˮ��������࣬���ý�ͷ�ιܼ�����ˮ����Һ�� ��Һ��������̶��� ���У���������������ݣ�
�ʴ�Ϊ��1-2����ͷ�ιܣ���Һ�棻���У�
��6������ƿ��û�п̶ȣ�ֻ�й̶��ݻ�������ƿ�����Բ����������������һ�����ʵ���Ũ�ȵ���Һ��
�ʴ�Ϊ������ƿ���ݻ��̶���
��7����Һ���������������ʣ��������ȴ�����£��ᵼ����Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ����Һ���������������ʣ��������ȴ�����£��ᵼ����Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
��8���ý�ͷ�ιܶ��ݺ���������Һ����ڿ̶���ʱ��Ҫ�ټ�ˮ��������Ϊ�ټ�ˮ������Һ�����ƫ��������Һ��Ũ��ƫС��
�ʴ�Ϊ���ټ�ˮ������Һ�����ƫ��������Һ��Ũ��ƫС��
��9�������ˮ����ʱ�����˿̶��ߣ����ܽ��������������ߣ�������������������ߵ������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ����������ƣ�
�ʴ�Ϊ���������ƣ�
��10�����ҡ��ʱ��С���������Σ������ټ�ˮ���̶ȣ������������ƣ���Ϊ�ټ�ˮ���̶ȵ������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ���ټ�ˮ���̶ȵ������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
��11���������ϴ��Һ��������ƿ�ᵼ�����ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ���������ϴ��Һ��������ƿ�ᵼ�����ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��5.3��
��2����������̼����5.3g��
�ʴ�Ϊ��5.3��
��3���ձ�ʢ�Ź���̼���ƣ���������������ܽ⣮
�ʴ�Ϊ���ձ�����������
��4��ת�ƣ����ձ��е���Һ�ز�����С�ĵ������� ����ƿ�У�
�ʴ�Ϊ��������������ƿ��
��5�����ݣ���������ƿ�������ˮʱ����̶��� 1-2cm��ֹͣ��Ϊ�����ˮ��������࣬���ý�ͷ�ιܼ�����ˮ����Һ�� ��Һ��������̶��� ���У���������������ݣ�
�ʴ�Ϊ��1-2����ͷ�ιܣ���Һ�棻���У�
��6������ƿ��û�п̶ȣ�ֻ�й̶��ݻ�������ƿ�����Բ����������������һ�����ʵ���Ũ�ȵ���Һ��
�ʴ�Ϊ������ƿ���ݻ��̶���
��7����Һ���������������ʣ��������ȴ�����£��ᵼ����Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ����Һ���������������ʣ��������ȴ�����£��ᵼ����Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
��8���ý�ͷ�ιܶ��ݺ���������Һ����ڿ̶���ʱ��Ҫ�ټ�ˮ��������Ϊ�ټ�ˮ������Һ�����ƫ��������Һ��Ũ��ƫС��
�ʴ�Ϊ���ټ�ˮ������Һ�����ƫ��������Һ��Ũ��ƫС��
��9�������ˮ����ʱ�����˿̶��ߣ����ܽ��������������ߣ�������������������ߵ������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ����������ƣ�
�ʴ�Ϊ���������ƣ�
��10�����ҡ��ʱ��С���������Σ������ټ�ˮ���̶ȣ������������ƣ���Ϊ�ټ�ˮ���̶ȵ������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ���ټ�ˮ���̶ȵ������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
��11���������ϴ��Һ��������ƿ�ᵼ�����ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ���������ϴ��Һ��������ƿ�ᵼ�����ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ������������Һ�����ѡȡ����ƿ�Ĺ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ʵ������Ҫ����400mL0.2mol/L����������Һ��ʵ�鲽������У�
ʵ������Ҫ����400mL0.2mol/L����������Һ��ʵ�鲽������У� F������õ���Һ�����Լ�ƿ�У����ϱ�ǩ����ϴ������ƿ��
F������õ���Һ�����Լ�ƿ�У����ϱ�ǩ����ϴ������ƿ��