��Ŀ����
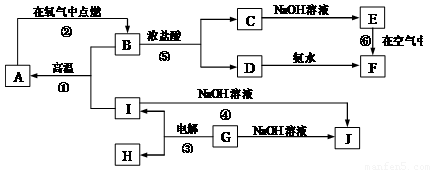
A-I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮�����ϵ����ͼ��ʾ�����ַ�Ӧ�������û���г�������֪HΪ����Ԫ�صĹ�̬�����F�Ǻ��ɫ������ˮ�ij�������A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�

����д���пհף�
��1��A��B��C��D��E��F����������������ͬһ��Ԫ����Ԫ�����ڱ���λ��__________
��2��д��C��H���ʵĻ�ѧʽ��C______��H_______
��3��д����Ӧ�١��Ļ�ѧ����ʽ�ۡ͢��ߵ����ӷ���ʽ��
��Ӧ�٣�________________________________
��Ӧ�ޣ�________________________________
��Ӧ�ۣ�________________________________
��Ӧ�ߣ�________________________________
��4����Ӧ�����е�������_____________��
��1��A��B��C��D��E��F����������������ͬһ��Ԫ����Ԫ�����ڱ���λ��__________
��2��д��C��H���ʵĻ�ѧʽ��C______��H_______
��3��д����Ӧ�١��Ļ�ѧ����ʽ�ۡ͢��ߵ����ӷ���ʽ��
��Ӧ�٣�________________________________
��Ӧ�ޣ�________________________________
��Ӧ�ۣ�________________________________
��Ӧ�ߣ�________________________________
��4����Ӧ�����е�������_____________��
��1���������ڵڢ���
��2��C��FeCl2��H��Al2O3
��3���� 8Al+3Fe3O4 4Al2O3+9Fe����4Fe(OH)2+O2+2H2O=4Fe(OH)3����Fe3O4+8H+=Fe2++2Fe3++4H2O����2Al+2OH-+2H2O=2AlO2-+3H2��
4Al2O3+9Fe����4Fe(OH)2+O2+2H2O=4Fe(OH)3����Fe3O4+8H+=Fe2++2Fe3++4H2O����2Al+2OH-+2H2O=2AlO2-+3H2��
��4���а�ɫ����Ѹ�ٱ�ɻ���ɫ�����ձ�Ϊ���ɫ
��2��C��FeCl2��H��Al2O3
��3���� 8Al+3Fe3O4
 4Al2O3+9Fe����4Fe(OH)2+O2+2H2O=4Fe(OH)3����Fe3O4+8H+=Fe2++2Fe3++4H2O����2Al+2OH-+2H2O=2AlO2-+3H2��
4Al2O3+9Fe����4Fe(OH)2+O2+2H2O=4Fe(OH)3����Fe3O4+8H+=Fe2++2Fe3++4H2O����2Al+2OH-+2H2O=2AlO2-+3H2����4���а�ɫ����Ѹ�ٱ�ɻ���ɫ�����ձ�Ϊ���ɫ

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
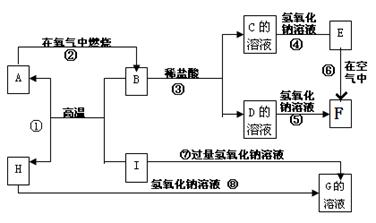
 A-I�ֱ��ʾ��ѧ��ѧ�еij������ʣ�����֮����ת����ϵ����ͼ��ʾ�����ַ�Ӧ�������û���г���������֪G��һ�����������A��B��C��D��E��F���������о�����ͬһ��Ԫ�أ�FΪ���ɫ������
A-I�ֱ��ʾ��ѧ��ѧ�еij������ʣ�����֮����ת����ϵ����ͼ��ʾ�����ַ�Ӧ�������û���г���������֪G��һ�����������A��B��C��D��E��F���������о�����ͬһ��Ԫ�أ�FΪ���ɫ������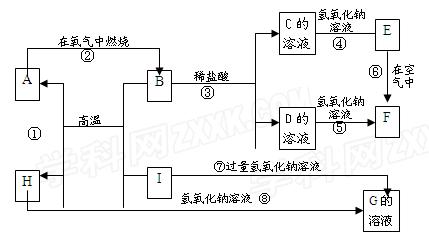
 _________________
_________________