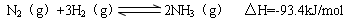��Ŀ����
Ϊ̽�����������H2(g)+I2(g)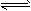 2HI (g)����H��0 ��Ӧ���ʵ�Ӱ�죬����±���ʾʵ��
2HI (g)����H��0 ��Ӧ���ʵ�Ӱ�죬����±���ʾʵ��
 2HI (g)����H��0 ��Ӧ���ʵ�Ӱ�죬����±���ʾʵ��
2HI (g)����H��0 ��Ӧ���ʵ�Ӱ�죬����±���ʾʵ��
��1������ϱ��еĿո�
��2��ʵ����У�I2�����ʵ���Ũ�ȣ�c����ʱ�䣨t���ı仯����ͼ��ʾ��
��2��ʵ����У�I2�����ʵ���Ũ�ȣ�c����ʱ�䣨t���ı仯����ͼ��ʾ��
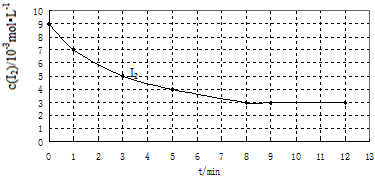
����ͼ�����ݻش��������⣺
����0��1min�ڷ�Ӧ��ƽ�����ʦͣ�HI��= _________��
���ڸ���������ͼ�л���ʵ�����I2�����ʵ���Ũ�ȣ�c����ʱ�䣨t���仯�����ߣ������б�Ҫ�ı�ע��
�۱��������ݻ����䣬�����м���1molH2����Ӧ����____________��ѡ��ӿ졢���������䡢��ȷ��������
�ܸ�ͬѧ�����ô˷�Ӧ�о�ѹǿ�Ի�ѧƽ���ƶ���Ӱ�죬�����ܷ�ﵽʵ��Ŀ�ģ� _________����ܡ������ܡ�����ԭ����__________________________
����0��1min�ڷ�Ӧ��ƽ�����ʦͣ�HI��= _________��
���ڸ���������ͼ�л���ʵ�����I2�����ʵ���Ũ�ȣ�c����ʱ�䣨t���仯�����ߣ������б�Ҫ�ı�ע��
�۱��������ݻ����䣬�����м���1molH2����Ӧ����____________��ѡ��ӿ졢���������䡢��ȷ��������
�ܸ�ͬѧ�����ô˷�Ӧ�о�ѹǿ�Ի�ѧƽ���ƶ���Ӱ�죬�����ܷ�ﵽʵ��Ŀ�ģ� _________����ܡ������ܡ�����ԭ����__________________________
��1��III�¶ȣ�457
��2����4��10-3mol��L-1��min-1
��2����4��10-3mol��L-1��min-1
��

�ۼӿ죻�ܲ��ܣ��÷�Ӧǰ�������������ȣ�ƽ�ⲻ��ѹǿ��Ӱ��

��ϰ��ϵ�д�
�����Ŀ
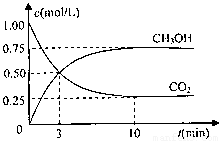
��ҵ���ڴ��������¿�����CO�ϳɼ״���CO(g)+2H2(g)  CH3OH(g)��ͼ1��ʾ��Ӧ�����������ı仯�����
CH3OH(g)��ͼ1��ʾ��Ӧ�����������ı仯�����

��ش��������⣺
��1����ͼI�У�����______(�a����b������ʾʹ���˴������÷�Ӧ����______(����ȡ����ȡ�����Ӧ��
��2��Ϊ̽����������Ժϳɼ״���ѧ��Ӧ���ʵ�Ӱ����ɣ�ij�Ƽ��������������������ʵ�飬�������������˱��У��벹��������
|
ʵ���� |
T/�� |
��ʼŨ��/mol•L��1 |
������������ �ȱ����/m2•g��1 |
|
|
CO |
H2 |
|||
|
�� |
280 |
1.20��10��3 |
5.80��10��3 |
82 |
|
�� |
280 |
1.20��10��3 |
5.80��10��3 |
124 |
|
�� |
350 |
|
|
124 |
�������ʵ����ٺ͢ڵ�Ŀ����______��
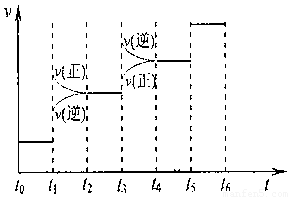
��3����ͼ2������ʾ�Ĺ����ǣ���p1ѹǿ��ƽ��ʱ______�����뻭����p2ѹǿ�£�p2 > p1)�����ߡ�
��4���ڼ��������¿ɽ��ϳɼ״��ķ�Ӧ��Ƴ�ԭ��أ����ĵ缫��ӦʽΪ______��
 ������������Ҫ���������ͻ�ԭ����������������ɱ����Ư�ȣ�ij��ѧ��ȤС��ͬѧΧ���Ź������չ�˵����о���ʵ�飮
������������Ҫ���������ͻ�ԭ����������������ɱ����Ư�ȣ�ij��ѧ��ȤС��ͬѧΧ���Ź������չ�˵����о���ʵ�飮
 CH3OH��g��+H2O��g����H=��49.0kJ/mol
CH3OH��g��+H2O��g����H=��49.0kJ/mol


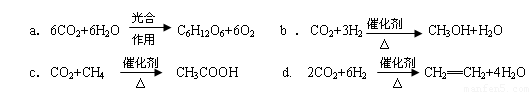
 CO2��g��+3H2��g�� CH3OH��g��+H2O��g����H=-49.0kJ/mol
CO2��g��+3H2��g�� CH3OH��g��+H2O��g����H=-49.0kJ/mol