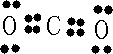��Ŀ����
��H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
Cu��s��+2H+��aq���TCu2+��aq��+H2��g����H=64.39kJ?mol-1
2H2O2��l���T2H2O��l��+O2��g����H=-196.46kJ?mol-1
H2��g��+
O2��g���TH2O��l����H=-285.84kJ?mol-1
�� H2SO4��Һ��Cu��H2O2��Ӧ����Cu2+��H2O���ȷ���ʽΪ ��
Cu��s��+2H+��aq���TCu2+��aq��+H2��g����H=64.39kJ?mol-1
2H2O2��l���T2H2O��l��+O2��g����H=-196.46kJ?mol-1
H2��g��+
| 1 | 2 |
�� H2SO4��Һ��Cu��H2O2��Ӧ����Cu2+��H2O���ȷ���ʽΪ
�����������Ȼ�ѧ����ʽ��˹���ɼ���õ������Ȼ�ѧ����ʽ��
����⣺��Cu��s��+2H+��aq���TCu2+��aq��+H2��g����H=+64.39kJ/mol��
��2H2O2��l���T2H2O��l��+O2��g����H=-196.46kJ/mol��
��H2��g��+
O2��g���TH2O��l����H=-285.84kJ/mol��
�ɸ�˹���ɢ�+
��+�۵õ���Cu��s��+H2O2��l��+2H+��aq���TCu2+��aq��+2H2O��l����H=-319.68kJ/mol��
�ʴ�Ϊ��Cu��s��+H2O2��l��+2H+��aq���TCu2+��aq��+2H2O��l����H=-319.68kJ/mol��
��2H2O2��l���T2H2O��l��+O2��g����H=-196.46kJ/mol��
��H2��g��+
| 1 |
| 2 |
�ɸ�˹���ɢ�+
| 1 |
| 2 |
�ʴ�Ϊ��Cu��s��+H2O2��l��+2H+��aq���TCu2+��aq��+2H2O��l����H=-319.68kJ/mol��
���������⿼�����Ȼ�ѧ����ʽ�ļ�����д����˹���ɵļ���Ӧ�ã���Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ