��Ŀ����
��Na2O2��NaHCO3�������������ܱ������м��ȳ�ַ�Ӧ���ų��������ȴ������ʱ���ܷ����ķ�Ӧ���£�
2Na2O2+2NaHCO3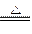 2NaOH+2Na2CO3+O2��
2NaOH+2Na2CO3+O2��
2Na2O2+4NaHCO3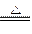 4Na2CO3+O2��+2H2O
4Na2CO3+O2��+2H2O
2NaHCO3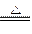 Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O
(1)���������������Ϊm����Ӧ����ȴ�ٳ������������˦�m����NaHCO3��Na2O2�����ʵ����ֱ�Ϊa��b����ȷ�����������¦�m��ȡֵ��Χ(�ú�m�Ĵ���ʽ��ʾ)��
�ٵ�a/b��1ʱ����mȡֵ��Χ��_____________________��
�ڵ�1<a/b��2ʱ����mȡֵ��Χ��___________________��
�۵�a/b>2ʱ����mȡֵ��Χ��______________________��
(2)��m="14.52" g����m=" 1.28" gʱ����Ӧ��IJ��������ڵμ�2.00 mol��L-1����140.0 mL���ɲ�����״���µ�����____________ L��
2Na2O2+2NaHCO3
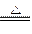 2NaOH+2Na2CO3+O2��
2NaOH+2Na2CO3+O2��2Na2O2+4NaHCO3
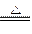 4Na2CO3+O2��+2H2O
4Na2CO3+O2��+2H2O2NaHCO3
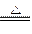 Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O(1)���������������Ϊm����Ӧ����ȴ�ٳ������������˦�m����NaHCO3��Na2O2�����ʵ����ֱ�Ϊa��b����ȷ�����������¦�m��ȡֵ��Χ(�ú�m�Ĵ���ʽ��ʾ)��
�ٵ�a/b��1ʱ����mȡֵ��Χ��_____________________��
�ڵ�1<a/b��2ʱ����mȡֵ��Χ��___________________��
�۵�a/b>2ʱ����mȡֵ��Χ��______________________��
(2)��m="14.52" g����m=" 1.28" gʱ����Ӧ��IJ��������ڵμ�2.00 mol��L-1����140.0 mL���ɲ�����״���µ�����____________ L��
(1)�٦�m��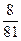 m
m
��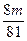 <��m��
<��m��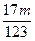
��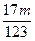 <��m<
<��m<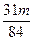
(2)2.016
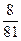 m
m��
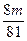 <��m��
<��m��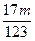
��
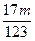 <��m<
<��m<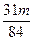
(2)2.016
(1)�ٵ�a/b��1ʱ����Ӧ����ʽ���У���m=m(O2)����
2Na2O2+2NaHCO3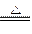 2NaOH+2Na2CO3+O2��
2NaOH+2Na2CO3+O2��
2��78+2��84 32
m ��m
��m��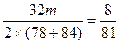 m
m
�ڵ�1<a/b��2ʱ����Ӧ����ʽ�����Ͻ�����ʽ���У���m=m(O2)+m(H2O)����
2Na2O2+4NaHCO3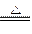 4Na2CO3+O2��+2H2O
4Na2CO3+O2��+2H2O
2��7 84��84 32+36
m ��m
���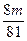 <��m��
<��m��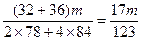
�۵�a/b>2ʱ��ͬ�������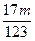 <��m<
<��m<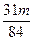 ��
��
(2)��Ϊ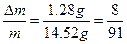 ����a/b<1�������Na2O2��������m=m(O2)��n(O2)=
����a/b<1�������Na2O2��������m=m(O2)��n(O2)=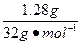 ="0.04" mol��
="0.04" mol��
2Na2O2+2NaHCO3 2NaOH + 2Na2CO3+O2��
2NaOH + 2Na2CO3+O2��
0.08 mol 0.08 mol 0.08 mol 0.04 mol
�������n(Na2O2)=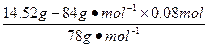 ="0.1" mol
="0.1" mol
��ˣ����������ڻ���n(Na2O2)="0.1" mol-0.08 mol="0.02" mol��
n(NaOH)="0.08" mol��n(Na2CO3)="0.08" mol���μ�n(HCl)="2.00" mol��L-1��0.140 L="0.280" molʱ�������ķ�Ӧ�У�
2Na2O2+2H2O====4NaOH+O2��
0.02 mol 0.04 mol 0.01 mol
NaOH + HCl====NaCl+H2O
(0.04+0.08) mol 0.12 mol
Na2CO3+HCl====NaCl+NaHCO3
0.08 mol 0.08 mol 0.08 mol
NaHCO3+HCl====NaCl+H2O+CO2��
0.08 mol 0.08 mol 0.08 mol
���ԣ��ɲ�����״���µ�����V="(0.08+0.01)" mol��22.4 L��mol-1="2.016" L��
2Na2O2+2NaHCO3
 2NaOH+2Na2CO3+O2��
2NaOH+2Na2CO3+O2��2��78+2��84 32
m ��m
��m��
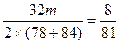 m
m�ڵ�1<a/b��2ʱ����Ӧ����ʽ�����Ͻ�����ʽ���У���m=m(O2)+m(H2O)����
2Na2O2+4NaHCO3
 4Na2CO3+O2��+2H2O
4Na2CO3+O2��+2H2O2��7 84��84 32+36
m ��m
���
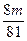 <��m��
<��m��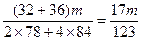
�۵�a/b>2ʱ��ͬ�������
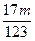 <��m<
<��m<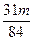 ��
��(2)��Ϊ
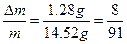 ����a/b<1�������Na2O2��������m=m(O2)��n(O2)=
����a/b<1�������Na2O2��������m=m(O2)��n(O2)=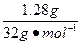 ="0.04" mol��
="0.04" mol��2Na2O2+2NaHCO3
 2NaOH + 2Na2CO3+O2��
2NaOH + 2Na2CO3+O2��0.08 mol 0.08 mol 0.08 mol 0.04 mol
�������n(Na2O2)=
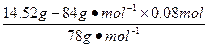 ="0.1" mol
="0.1" mol��ˣ����������ڻ���n(Na2O2)="0.1" mol-0.08 mol="0.02" mol��
n(NaOH)="0.08" mol��n(Na2CO3)="0.08" mol���μ�n(HCl)="2.00" mol��L-1��0.140 L="0.280" molʱ�������ķ�Ӧ�У�
2Na2O2+2H2O====4NaOH+O2��
0.02 mol 0.04 mol 0.01 mol
NaOH + HCl====NaCl+H2O
(0.04+0.08) mol 0.12 mol
Na2CO3+HCl====NaCl+NaHCO3
0.08 mol 0.08 mol 0.08 mol
NaHCO3+HCl====NaCl+H2O+CO2��
0.08 mol 0.08 mol 0.08 mol
���ԣ��ɲ�����״���µ�����V="(0.08+0.01)" mol��22.4 L��mol-1="2.016" L��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 �Ĺ���������������в���ȷ����( )
�Ĺ���������������в���ȷ����( ) ��2-
��2-