��Ŀ����
����������;�㷺������Ư������Ӱ�����������ȣ�����������SO2��NH3��NaClΪԭ��������ˮ�������Ƶ��·����õ���չ������������ͼ1��ʾ��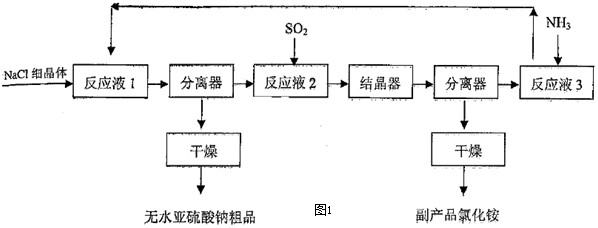
��1����ѧ��ѧʵ����ʵ�����������С������������õIJ��������ǣ�
��2������ӦҺ1������SO2ͨ��14.8%��ˮ�γɵ����������Һ����60�桫80��ʱ�����Ͻ��裬����NaClϸ���壬������ˮ��������������������ˮ�������Ƶ����ӷ�Ӧ����ʽΪ
��3���ڡ���ӦҺ2��ͨ������SO2����Һ�е������ӳ�OH-���
��4��������������ԭ�������ʣ�ÿ��100g��ˮ�������ƣ������ʵ�������������Ʒ�����������
| ԭ�� | ����ֵ | ʵ��ֵ | ������ |
| SO2 | 50.79 | 54.4 | 93.36% |
| NaCl | 92.86 | 101.0 | 91.94% |
| NH3 | 26.98 | 30.5 | 88.46% |
| ����ƷNH4Cl | a | 87.1 | -------- |
��5����ˮ�������ƴ�Ʒ�����ؽᾧ�ķ������о��ƣ���������ʵ������ѭ���ľ����������Ʋ���������ͼ����ͼ2��ʾ����
��������1�������С��������������Ƿ�������Һ�壬���ݹ��˲�������������
��2�����������NaCl���ɾ�Na2SO3�壻
��3��ͨ������SO2����������������ɣ��ڡ���ӦҺ3����ͨ������NH3��HSO3-ת��ΪSO32-��
��4�����ݷ�Ӧ����ʽ����������NH4Cl�IJ�����
��5�������ؽᾧ��ʵ�����������
��2�����������NaCl���ɾ�Na2SO3�壻
��3��ͨ������SO2����������������ɣ��ڡ���ӦҺ3����ͨ������NH3��HSO3-ת��ΪSO32-��
��4�����ݷ�Ӧ����ʽ����������NH4Cl�IJ�����
��5�������ؽᾧ��ʵ�����������
����⣺��1�������С��������������Ƿ�������Һ�壬���Բ��������ǣ����ˣ����ݹ��˲�����֪���貣��������©�����ձ�����������
�ʴ�Ϊ�����ˣ�©�����ձ�����������
��2�����������NaCl���ɾ�Na2SO3�壬����������ˮ�������Ƶ����ӷ�Ӧ����ʽΪ2Na++SO32-=Na2SO3����
�ʴ�Ϊ��2Na++SO32-=Na2SO3����
��3��ͨ������SO2����������������ɣ���֪ԭ��Һ����Cl-�������ڡ���ӦҺ2��ͨ������SO2����Һ�е������ӳ�OH-���HSO3-��Cl-���ڡ���ӦҺ3����ͨ������NH3��HSO3-ת��ΪSO32-��Ȼ��ѭ��������ӦҺ1����NaCl��Ӧ������ˮ�������ƾ��壻
�ʴ�Ϊ��HSO3-��Cl-�����䰱ʹ��Һ�е�HSO3-ת��ΪSO32-��
��4������NH4Cl�ķ�Ӧ����Ϊ����NH4��2SO3+2NaCl=Na2SO3��+2NH4Cl����֪����NaCl������ֵΪ92.86g��
�� NaCl��NH4Cl
58.5 53.5
92.86g ag
��
=
�����a=84.92g�����ڲ�Ʒ�п��ܺ������ʣ���������ֵ��ʵ��ֵҪС��
�ʴ�Ϊ��84.92g��NH4Clʵ��ֵƫ�ߣ����ܲ�Ʒ�к������ʣ�
��5���ؽᾧ��ʵ�����Ϊ������Ʒ��ˮ�ܽ⣬Ȼ������Ũ������ȴ�ᾧ���ٹ��ˣ�����õ��ϴ��ľ��壬���Ծ����������Ʋ���������ͼΪ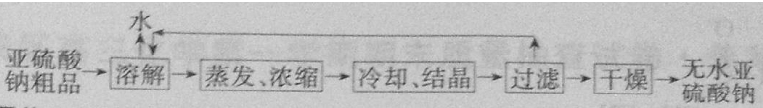
�ʴ�Ϊ�� ��
��
�ʴ�Ϊ�����ˣ�©�����ձ�����������
��2�����������NaCl���ɾ�Na2SO3�壬����������ˮ�������Ƶ����ӷ�Ӧ����ʽΪ2Na++SO32-=Na2SO3����
�ʴ�Ϊ��2Na++SO32-=Na2SO3����
��3��ͨ������SO2����������������ɣ���֪ԭ��Һ����Cl-�������ڡ���ӦҺ2��ͨ������SO2����Һ�е������ӳ�OH-���HSO3-��Cl-���ڡ���ӦҺ3����ͨ������NH3��HSO3-ת��ΪSO32-��Ȼ��ѭ��������ӦҺ1����NaCl��Ӧ������ˮ�������ƾ��壻
�ʴ�Ϊ��HSO3-��Cl-�����䰱ʹ��Һ�е�HSO3-ת��ΪSO32-��
��4������NH4Cl�ķ�Ӧ����Ϊ����NH4��2SO3+2NaCl=Na2SO3��+2NH4Cl����֪����NaCl������ֵΪ92.86g��
�� NaCl��NH4Cl
58.5 53.5
92.86g ag
��
| 58.5 |
| 92.86g |
| 53.5 |
| ag |
�ʴ�Ϊ��84.92g��NH4Clʵ��ֵƫ�ߣ����ܲ�Ʒ�к������ʣ�
��5���ؽᾧ��ʵ�����Ϊ������Ʒ��ˮ�ܽ⣬Ȼ������Ũ������ȴ�ᾧ���ٹ��ˣ�����õ��ϴ��ľ��壬���Ծ����������Ʋ���������ͼΪ

�ʴ�Ϊ��
 ��
�����������⿼���˹�������ͼ���漰������ʵ�������������ѡ����ʽ����д�����۲����ļ���ȣ���Ŀ�漰�����ݽ϶࣬�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ