��Ŀ����
��ij��Һ������Fe2+��Cu2+��Cl-���л���ͭ���Ƶô�����FeCl3��Һ�������Ƶô�����FeCl3��ҺΪԭ����ȡ������ˮ�������������(K2FeO4)�����������£�

��֪���������(K2FeO4)λ����ɫ���壬������ˮ�������Ի�������Һ���ֽ⣬�ڼ�����Һ���ȶ���������ؾ���ǿ�����ԡ��������(K2FeO4)��ˮ���ò���Fe(OH)3��
��1�������Һ�к���Fe2+ѡ�õ��Լ���________���ѧʽ�����ӷ�Һ���Ƶô�����FeCl3��Һ������Լ��������⣬����Ҫһ���Լ���_________���ѧʽ��������ʱ������Ӧ�����ӷ���ʽΪ__________��
��2���������(K2FeO4)�ڴ���ˮ�����е�������_________��__________��
��3�������������̷�Ӧ�����ӷ���ʽΪ__________��
��4������ʱ���õIJ��������в��������ձ���________���������յõ��ĸ�����س�������־�������ؽᾧ���ᴿ�������ǣ����ֲ�Ʒ��_________�ܽ⣬Ȼ��________��
��5���û��յ�ͭΪԭ�Ͽ��Ƶô���CuSO4��5H2O���壨����������FeSO4��7H2O������ȥCuSO4��5H2O��������־�ķ����ǣ�����Һ�м���H2O2���ٵ�����ҺPH�����˼����Ƶô�����CuSO4��Һ�������Ƶô���CuSO4��5H2O�ľ��塣
��֪������ʱһЩ���ʵ�Kaq���±���
��ѧʽ | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 |
Kaq | 8.0��10-16 | 8.0��10-18 | 8.0��10-20 |
��֪��Һ�е�����Ũ��С��1��10-6mol��L-1ʱ���϶�������ȫ��
��˫��ˮ��Ŀ����__________������Һ��CuSO4��Ũ��Ϊ3.0mol��L-1��ͨ������˵���˷����ɳ�ȥ����CuSO4��5H2O������FeSO4��7H2O������________��
 ����������ϵ�д�
����������ϵ�д�

 ij����A��һ�������¼��ȷֽ⣬���ﶼ�����壬�ֽⷽ��ʽΪ2A
ij����A��һ�������¼��ȷֽ⣬���ﶼ�����壬�ֽⷽ��ʽΪ2A B+2C+2D��������ɵĻ�����������������ܶ�Ϊd����A����Է�������Ϊ
B+2C+2D��������ɵĻ�����������������ܶ�Ϊd����A����Է�������Ϊ

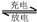
 �ݴ˷�Ӧʽ�жϣ����������в���ȷ����
�ݴ˷�Ӧʽ�жϣ����������в���ȷ����
 +2
+2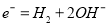

 00%����ԭ�������_______________��������������ȤС�������(Ce)�����ⶨ��Ʒ��Fe2+�ĺ�����ȡ2.880 g��Ʒ���100 mL��Һ��ÿ��ȡ20.00 mL�����б�Ҫ��������0.100 0 mol��L-1Ce(SO4)2����Һ�ζ����յ㣬ƽ������Ce(SO4)219.7 mL���ζ���ӦΪCe4++Fe2+====Ce3++Fe3+�����Ʒ�����������������������Ϊ____________��
00%����ԭ�������_______________��������������ȤС�������(Ce)�����ⶨ��Ʒ��Fe2+�ĺ�����ȡ2.880 g��Ʒ���100 mL��Һ��ÿ��ȡ20.00 mL�����б�Ҫ��������0.100 0 mol��L-1Ce(SO4)2����Һ�ζ����յ㣬ƽ������Ce(SO4)219.7 mL���ζ���ӦΪCe4++Fe2+====Ce3++Fe3+�����Ʒ�����������������������Ϊ____________��