��Ŀ����
ͨ��״���£�X��Y��Z��������̬���ʡ�X�����Ԫ���ǵ�������ԭ�Ӱ뾶��С��Ԫ��(ϡ������Ԫ�س���)��Y��Z����Ԫ��R��ɣ���ӦY+2I-+2H+====I2+Z+H2O����ΪY�ļ�����Ӧ��
(1)Y��Z�Ĺ�ϵ��(ѡ����ĸ)______________________��
a.ͬλ�� b.ͬϵ��
c.ͬ�������� d.ͬ���칹��
(2)��Y�Ͷ�������ֱ�ͨ��Ʒ����Һ������ʹƷ����ɫ����������ɫ����Һ������ߵ�ʵ�鷽��:________________________________________________________��
(3)�ٳ�ʵ��˵��X�������Ա����ʵ�������ǿ(�û�ѧ����ʽ��ʾ):________________��
(4)����(CN)2��X��ѧ�������ƣ�Ҳ����H2��Ӧ����HCN(��ˮ��Һ��һ����)��
��HCN�����к���4�����ۼ�����ṹʽ��___________________________��
��KCN��Һ�Լ��ԣ�ԭ����(�����ӷ���ʽ��ʾ)____________________________________��
(5)���������£�������Z��ij����M����MCR3(CΪ̼Ԫ��)��ȫ��Ӧ����CR2��MmRn(m��n��Ϊ������)����CR2����Ϊw1 g��MmRn����Ϊw2 g��M�����ԭ������Ϊa����MmRn��m��n��______________________(�ú�w1��w2��a�Ĵ���ʽ��ʾ)��
(1)Y��Z�Ĺ�ϵ��(ѡ����ĸ)______________________��
a.ͬλ�� b.ͬϵ��
c.ͬ�������� d.ͬ���칹��
(2)��Y�Ͷ�������ֱ�ͨ��Ʒ����Һ������ʹƷ����ɫ����������ɫ����Һ������ߵ�ʵ�鷽��:________________________________________________________��
(3)�ٳ�ʵ��˵��X�������Ա����ʵ�������ǿ(�û�ѧ����ʽ��ʾ):________________��
(4)����(CN)2��X��ѧ�������ƣ�Ҳ����H2��Ӧ����HCN(��ˮ��Һ��һ����)��
��HCN�����к���4�����ۼ�����ṹʽ��___________________________��
��KCN��Һ�Լ��ԣ�ԭ����(�����ӷ���ʽ��ʾ)____________________________________��
(5)���������£�������Z��ij����M����MCR3(CΪ̼Ԫ��)��ȫ��Ӧ����CR2��MmRn(m��n��Ϊ������)����CR2����Ϊw1 g��MmRn����Ϊw2 g��M�����ԭ������Ϊa����MmRn��m��n��______________________(�ú�w1��w2��a�Ĵ���ʽ��ʾ)��
(1)c
(2)������ɫ�����Һ������Һ�ָ���ɫ����ԭͨ������ΪSO2������Һ����죬��ԭͨ��������O3
(3)2Fe+3Cl2 2FeCl3��Fe+S
2FeCl3��Fe+S FeS(���������𰸾���)
FeS(���������𰸾���)
(4)��H��C��N
��CN-+H2O HCN+OH-
HCN+OH-
(5)16w1��(44w2-a��1)
(2)������ɫ�����Һ������Һ�ָ���ɫ����ԭͨ������ΪSO2������Һ����죬��ԭͨ��������O3
(3)2Fe+3Cl2
 2FeCl3��Fe+S
2FeCl3��Fe+S FeS(���������𰸾���)
FeS(���������𰸾���)(4)��H��C��N
��CN-+H2O
 HCN+OH-
HCN+OH-(5)16w1��(44w2-a��1)
X�����Ԫ���ǵ�������ԭ�Ӱ뾶��С��Ԫ��(ϡ������Ԫ�س���),X��Cl2����ӦY+2I-+2H+====I2+Z+H2O��Y������Ԫ����ɵĵ��ʣ�������O3��O2,�ܷ������淴Ӧ��Y��O3��Z��O2��(1)��ͬһԪ����ɵ����ʲ�ͬ�ļ��ֵ��ʽ���ͬ�������塣(2)SO2��O3������Ư���ԣ�����Ư��ԭ����ͬ��SO2�����л�ɫ�ʷ������϶���ɫ������ʱ�Եģ����Ȼ�ָ�ԭ�е�Ʒ����ɫ����O3�Ƿ���ǿ������ԭ��Ӧ����ɫ����������ɫ�����ָ���(3)�Ƚ�������ǿ���ķ����ܶ࣬�û�����ͬ������Ӧ��̬�ĸߵ͵ȡ�(4)���û�ѧ��֪ʶ���Եõ��𰸡�KCN��Һ�Լ���������ˮ������H+����OH-���£��õ�CN-+H2O HCN+OH-��
HCN+OH-��
(5)2mMCO3��(n��m)O2 2mCO2��2MmOn
2mCO2��2MmOn
w1 g w2 g
��ʽ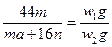 ,���Ϊ16w1��(44w2-aw1)��
,���Ϊ16w1��(44w2-aw1)��
 HCN+OH-��
HCN+OH-��(5)2mMCO3��(n��m)O2
 2mCO2��2MmOn
2mCO2��2MmOnw1 g w2 g
��ʽ
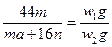 ,���Ϊ16w1��(44w2-aw1)��
,���Ϊ16w1��(44w2-aw1)��
��ϰ��ϵ�д�
 �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�����Ŀ
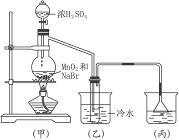
 Na2SO4+HSCN��
Na2SO4+HSCN��