��Ŀ����
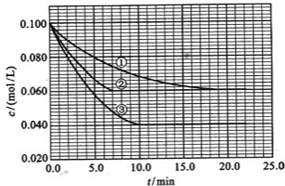
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������W������������Ӧ��ˮ�����Ũ��Һ����Cu��Ӧ�������в���ȼ�����壬��ش���������
��1��Ԫ��Q��W�ķǽ�����ǿ����Q_____W���>����<������
��2��д���ڼ��������£�����R������������Ӧ��ˮ����Y��Ũ��Һ��Fe��Ӧ��������Ψһ�����������V�ĵ��ʰ������ʵ���֮��4:1�������ȫ����ˮ�������ӷ���ʽ��_________________________��
��3����mg����Cu����ȫ�ܽ���80mL13.5mol/L��Y��Һ�У��ռ���6.72L����������壬ʣ����Һ��H+Ũ��Ϊ1mol/L�����跴Ӧ����Һ�����Ϊ80mL����m=_________g��
��2��д���ڼ��������£�����R������������Ӧ��ˮ����Y��Ũ��Һ��Fe��Ӧ��������Ψһ�����������V�ĵ��ʰ������ʵ���֮��4:1�������ȫ����ˮ�������ӷ���ʽ��_________________________��
��3����mg����Cu����ȫ�ܽ���80mL13.5mol/L��Y��Һ�У��ռ���6.72L����������壬ʣ����Һ��H+Ũ��Ϊ1mol/L�����跴Ӧ����Һ�����Ϊ80mL����m=_________g��
��1��Q < W
��2��Fe+3NO3-+6H+==Fe3++3NO2��+3H2O
��3��22.4 g
��2��Fe+3NO3-+6H+==Fe3++3NO2��+3H2O
��3��22.4 g

��ϰ��ϵ�д�
�����Ŀ
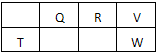
 ��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
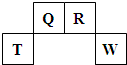
 ��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������
��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������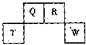 ��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺