��Ŀ����
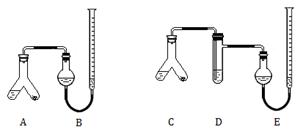
 ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ��
ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ���ṩ���Լ�����ϸ�ĸ�Ƭ��ĩ ����Ƭ�е������ɷֲ������ᷴӦ����2mol?L-1���ᡢ5% NaOH��Һ������Na2CO3��Һ������NaHCO3��Һ������ˮ��
ʵ����̣�
�������װ�õ������ԣ�
����A��C���ұ���0.25g��Ƭ��ĩ�������3mL 2mol?L-1���ᣬ�������ӣ���B��E�о����뱥��NaHCO3��Һ����ͼ��ʾ�����������ܶ�����
��A��C��б��ʹҺ��������ϣ�ʵ���������ȴ����������ܶ��������B���ռ���������Ϊ41.90mL��E���ռ������������Ϊ39.20mL���������������������Ϊ��״���µ��������
�ش��������⣺
��1�����м��ͼ1װ�������Եķ�����
��B���Ҳ��������м�ˮ��һ��ʱ�������װ������Һ���䣬˵������������
��B���Ҳ��������м�ˮ��һ��ʱ�������װ������Һ���䣬˵������������
����2��A�з�����Ӧ�����ӷ���ʽΪ
CaCO3+2H+=Ca2++CO2��+H2O
CaCO3+2H+=Ca2++CO2��+H2O
��D�м����Լ�Ϊ����ˮ
����ˮ
��D�����������ջӷ��������Ȼ���
���ջӷ��������Ȼ���
����3��ʵ��ǰ��������Һ����ͬһˮƽ���ϣ�������ʱ�ҹܵ�Һ�������ܵ�Һ�棬Ӧ���еIJ�����
�����ƶ��ҹܣ�ʹ��������Һ����ƽ
�����ƶ��ҹܣ�ʹ��������Һ����ƽ
����4��ͼ2ʵ�����ø�Ƭ�е�̼��Ƶ���������Ϊ
70%
70%
��ͼ1ʵ���ͼ 2ʵ�����ø�Ƭ�е�̼��ƺ���ƫ�ߣ������ӷ���ʽ��ʾƫ�ߵ�ԭ��HCO3-+H+=CO2��+H2O
HCO3-+H+=CO2��+H2O
����������1��װ������������װ���������ѹǿ�仯��Һ��仯�����жϣ�
��2��A����̼��ƺ�����ķ�Ӧ��Ӧ��D����Ϊ�������Ȼ����������Ӱ�������̼�IJⶨ�����
��3��Ӧ�����ƶ��ҹܣ�ʹ��������Һ����ͬһˮƽ���ϣ�
��4���������ɵĶ�����̼��������������ʵ�������Ϸ�Ӧ���ӷ���ʽ����̼������ʵ������õ�̼�������������ͼ1ʵ���ͼ2ʵ�����ø�Ƭ�е�̼��ƺ���ƫ������Ϊͼ1�����ɵĶ�����̼�����к����Ȼ��⣬��̼�����Ʒ�Ӧ�������˶�����̼��
��2��A����̼��ƺ�����ķ�Ӧ��Ӧ��D����Ϊ�������Ȼ����������Ӱ�������̼�IJⶨ�����
��3��Ӧ�����ƶ��ҹܣ�ʹ��������Һ����ͬһˮƽ���ϣ�
��4���������ɵĶ�����̼��������������ʵ�������Ϸ�Ӧ���ӷ���ʽ����̼������ʵ������õ�̼�������������ͼ1ʵ���ͼ2ʵ�����ø�Ƭ�е�̼��ƺ���ƫ������Ϊͼ1�����ɵĶ�����̼�����к����Ȼ��⣬��̼�����Ʒ�Ӧ�������˶�����̼��
����⣺��1�������м��ͼ1װ�������Եķ���������װ���е�����ѹǿ�仯���з�������B���Ҳ��������м�ˮ��һ��ʱ�������װ������Һ���䣬˵�����������ã�
�ʴ�Ϊ����B���Ҳ��������м�ˮ��һ��ʱ�������װ������Һ���䣬˵�����������ã�
��2��A����̼��ƺ�����ķ�Ӧ��Ӧ����Ӧ�����ӷ���ʽΪ��CaCO3+2H+�TCa2++CO2��+H2O��D����Ϊ�������Ȼ����������Ӱ�������̼�IJⶨ�����������ˮ���գ�
�ʴ�Ϊ��CaCO3+2H+�TCa2++CO2��+H2O������ˮ�����ջӷ��������Ȼ��⣻
��3������ʱ�ҹܵ�Һ�������ܵ�Һ�棬Ӧ�����ƶ��ҹܣ�ʹ��������Һ����ͬһˮƽ���ϣ�
�ʴ�Ϊ�������ƶ��ҹܣ�ʹ��������Һ����ƽ��
��4����A��C���ұ���0.25g��Ƭ��ĩ�������3mL 2mol/L���ᣬE���ռ������������Ϊ39.20mL���ʵ���=
=0.00175mol������̼��Ƶ�����=0.00175mol��100g/mol=0.175g��̼�����������=
��100%=70%��ͼ1�����ɵĶ�����̼�����к����Ȼ��⣬��̼�����Ʒ�Ӧ�������˶�����̼ʹ�ⶨ���ƫ�ߣ���Ӧ�����ӷ���ʽΪ��HCO3-+H+�TCO2��+H2O��
�ʴ�Ϊ��70%��HCO3-+H+�TCO2��+H2O��
�ʴ�Ϊ����B���Ҳ��������м�ˮ��һ��ʱ�������װ������Һ���䣬˵�����������ã�
��2��A����̼��ƺ�����ķ�Ӧ��Ӧ����Ӧ�����ӷ���ʽΪ��CaCO3+2H+�TCa2++CO2��+H2O��D����Ϊ�������Ȼ����������Ӱ�������̼�IJⶨ�����������ˮ���գ�
�ʴ�Ϊ��CaCO3+2H+�TCa2++CO2��+H2O������ˮ�����ջӷ��������Ȼ��⣻
��3������ʱ�ҹܵ�Һ�������ܵ�Һ�棬Ӧ�����ƶ��ҹܣ�ʹ��������Һ����ͬһˮƽ���ϣ�
�ʴ�Ϊ�������ƶ��ҹܣ�ʹ��������Һ����ƽ��
��4����A��C���ұ���0.25g��Ƭ��ĩ�������3mL 2mol/L���ᣬE���ռ������������Ϊ39.20mL���ʵ���=
| 0.0392L |
| 22.4L/mol |
| 0.175g |
| 0.25g |
�ʴ�Ϊ��70%��HCO3-+H+�TCO2��+H2O��
���������⿼����ʵ��װ�õķ����жϡ��������ʵķ���Ӧ�á�ʵ����Ƶķ����ͷ�Ӧ���������Ӧ�ã�ע��ʵ��ʱ�Ķ�����Ϊ�״��㣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ