��Ŀ����
��12�֣������ᴿ���о���
���й����ϡ�
| ��ѧʽ | CaCO3 | CaSO3 | CaC2O4 | Mg(OH)2 |
| Ksp | 4.96��10��9 | 4.96��10��9 | 2.34��10��9 | 5.61��10��12 |
ij�о���ѧϰС��Դ��ε��ᴿ�ͼ�������о��������һЩ�µķ�������֪�ô�����Ʒ����Ҫ���в��������ʡ�Mg2+��Ca2+�ȣ�����SO42���Ĵ��ڣ�����С������������£�
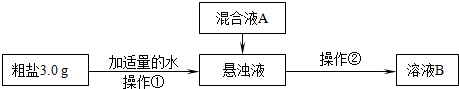
 ����Ƴ��ӹ��̡�
����Ƴ��ӹ��̡�
��1������������Ҫʹ�õIJ��������� �� �������ڵ�����Ϊ ��
��2�����ҺA����Ҫ�ɷ��� �����ѧʽ��
�������������
��3��Ϊ������ҺB��Mg2+��Ca2+�Ƿ������ͨ���ֱ�ȡ������ҺB����֧�Թ��У���������ʵ�飺
����һ������Mg2+�Ƿ������������һ֧�Թ��м��� ��Һ���ѧʽ�������Ƿ��г������ɡ�
�����������Ca2+�Ƿ����������һ֧�Թ��м���ij��Һ�����Ƿ��г������ɡ�Ч����õ��� ������ĸ����A��Na2CO3 B��Na2SO3 C��Na2C2O4
����ȡ����ʳ�Ρ�
��4������ҺB���Ȳ����ϵμ�6 mol��L��1��������Һ��ͬʱ��pH��ֽ�����Һ��ֱ��pH=2ʱֹͣ�����ᣬ�õ���ҺC���ò�����Ŀ���� ��
��5������ҺC���� �����������ƣ��У������������ò��������Ͻ��裬ֱ�� ʱ��������ֹͣ���ȡ�
���������ۡ�
��6���ڳ��ӹ����У����������Һ�мӻ��ҺA���ò����п�����ҺpH=12��ȷ��Mg2+�����������ṩ�����ݼ��㣬��ҺB��Mg2+���ʵ���Ũ�Ƚ��������� ���¡�
��12�֣�
��1���ձ��������������� ��2�֣�
��2��NaOH��Na2CO3 ��2�֣�
��3��NaOH C ��2�֣�
��4����ȥNaOH��Na2CO3 ��2�֣�
��5�������� ���������д�������������2�֣�
��6��5.61��10��8 mol��L��1����2�֣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д������ᴿ���о���
���й����ϡ�
| ��ѧʽ | CaCO3 | CaSO3 | CaC2O4 | Mg��OH��2 |
| Ksp | 4.96��10-9 | 4.96��10-9 | 2.34��10-9 | 5.61��10-12 |
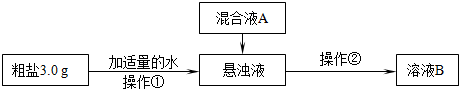
����Ƴ��ӹ��̡�
��1������������Ҫʹ�õIJ���������______��______�������ڵ�����Ϊ______�����ڲ����ڽ���������ҺB���л��ǣ�Ӧ��ȡ�Ĵ�ʩ��______��
��2�����ҺA����Ҫ�ɷ���______�����ѧʽ��
�������������
��3��Ϊ������ҺB��Mg2+��Ca2+�Ƿ������ͨ���ֱ�ȡ������ҺB����֧�Թ��У���������ʵ�飺
����һ������Mg2+�Ƿ������������һ֧�Թ��м���______��Һ���ѧʽ�������Ƿ��г������ɣ�
�����������Ca2+�Ƿ����������һ֧�Թ��м���ij��Һ�����Ƿ��г������ɣ�Ч����õ���______������ĸ����
A��Na2CO3������ B��Na2SO3��������C��Na2C2O4
����ȡ����ʳ�Ρ�
��4������ҺB���Ȳ����ϵμ�6mol?L-1��������Һ��ͬʱ��pH��ֽ�����Һ��ֱ��pH=5ʱֹͣ�����ᣬ�õ���ҺC���ò�����Ŀ����______��
��5������ҺC����______�����������ƣ��У������������ò��������Ͻ��裬ֱ��______ʱ��������ֹͣ���ȣ�
���������ۡ�
��6���ڳ��ӹ����У����������Һ�мӻ��ҺAʱ��Ҫ���ȣ�Ŀ����______���ò����п�����ҺpH=12��ȷ��Mg2+�����������ṩ�����ݼ��㣬��ҺB��Mg2+���ʵ���Ũ�Ƚ���������______���£�
�����ᴿ���о���
![]() ���й����ϡ�
���й����ϡ�
��ѧʽ | CaCO3 | CaSO3 | CaC2O4 | Mg(OH)2 |
Ksp | 4.96��10�D9 | 4.96��10�D9 | 2.34��10�D9 | 5.61��10�D12 |
ij�о���ѧϰС��Դ��ε��ᴿ�ͼ�������о��������һЩ�µķ�������֪�ô�����Ʒ����Ҫ���в��������ʡ�Mg2+��Ca2+��(����SO42�D�Ĵ���),��С������������£�
![]()

����Ƴ��ӹ��̡�
![]()
![]()
(1)����������Ҫʹ�õIJ��������� �� �������ڵ�����Ϊ �����ڲ����ڽ���������ҺB���л��ǣ�Ӧ��ȡ�Ĵ�ʩ��_ _____________________________________________________��
(2)���ҺA����Ҫ�ɷ���____________________��(�ѧʽ)
�������������
(3)Ϊ������ҺB��Mg2+��Ca2+�Ƿ������ͨ���ֱ�ȡ������ҺB����֧�Թ��У���������ʵ�飺
����һ������Mg2+�Ƿ������������һ֧�Թ��м��� ��Һ(�ѧʽ)�����Ƿ��г������ɡ�
�����������Ca2+�Ƿ����������һ֧�Թ��м���ij��Һ�����Ƿ��г������ɡ�Ч����õ��� (����ĸ)��
A��Na2CO3 B��Na2SO
����ȡ����ʳ�Ρ�
(4)����ҺB���Ȳ����ϵμ�6 mol?L�D1��������Һ��ͬʱ��pH��ֽ�����Һ��ֱ��pH=5ʱֹͣ�����ᣬ�õ���ҺC���ò�����Ŀ���� ��
(5)����ҺC���� (����������)�У������������ò��������Ͻ��裬ֱ�� ![]() ___________ _____________ʱ(������)��ֹͣ���ȡ�
___________ _____________ʱ(������)��ֹͣ���ȡ�
���������ۡ�
(6)�ڳ��ӹ����У����������Һ�мӻ��ҺAʱ��Ҫ���ȣ�Ŀ���� ���ò����п�����ҺpH=12��ȷ��Mg2+�����������ṩ�����ݼ��㣬��ҺB��Mg2+���ʵ���Ũ�Ƚ���������______________���¡�
���й����ϡ�
| ��ѧʽ | CaCO3 | CaSO3 | CaC2O4 | Mg��OH��2 |
| Ksp | 4.96×10-9 | 4.96×10-9 | 2.34×10-9 | 5.61×10-12 |
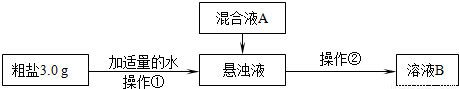
����Ƴ��ӹ��̡�
��1������������Ҫʹ�õIJ���������______��______�������ڵ�����Ϊ______�����ڲ����ڽ���������ҺB���л��ǣ�Ӧ��ȡ�Ĵ�ʩ��______��
��2�����ҺA����Ҫ�ɷ���______�����ѧʽ��
�������������
��3��Ϊ������ҺB��Mg2+��Ca2+�Ƿ������ͨ���ֱ�ȡ������ҺB����֧�Թ��У���������ʵ�飺
����һ������Mg2+�Ƿ������������һ֧�Թ��м���______��Һ���ѧʽ�������Ƿ��г������ɣ�
�����������Ca2+�Ƿ����������һ֧�Թ��м���ij��Һ�����Ƿ��г������ɣ�Ч����õ���______������ĸ����
A��Na2CO3 B��Na2SO3 C��Na2C2O4
����ȡ����ʳ�Ρ�
��4������ҺB���Ȳ����ϵμ�6mol?L-1��������Һ��ͬʱ��pH��ֽ�����Һ��ֱ��pH=5ʱֹͣ�����ᣬ�õ���ҺC���ò�����Ŀ����______��
��5������ҺC����______�����������ƣ��У������������ò��������Ͻ��裬ֱ��______ʱ��������ֹͣ���ȣ�
���������ۡ�
��6���ڳ��ӹ����У����������Һ�мӻ��ҺAʱ��Ҫ���ȣ�Ŀ����______���ò����п�����ҺpH=12��ȷ��Mg2+�����������ṩ�����ݼ��㣬��ҺB��Mg2+���ʵ���Ũ�Ƚ���������______���£�