��Ŀ����
( 10�֣���֪��
�ױ�����±�������л������Ļ���ԭ�ϣ���������������ijЩ���ϡ�ҩ�P�㷺Ӧ����ӡˢ�����ӹ�ҵ�еĸй���֬����Ҫ�м��壬����֮��Ĺ�ϵ����ͼ��ʾ��
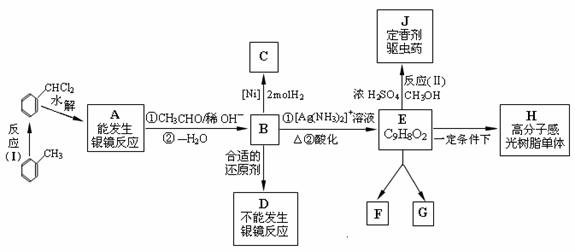
ͼ�� B��D��E ����ʹ��ˮ��ɫ�� E �ڹ�ȷ����������¿��Է������ۼӳɷ�Ӧ�� ���ɻ�Ϊͬ���칹��Ļ�״������ F �� G�� E ��������һ���������γ�һ�����ʸ߷��Ӹй���֬�ĵ���H��
��ش��������⣺
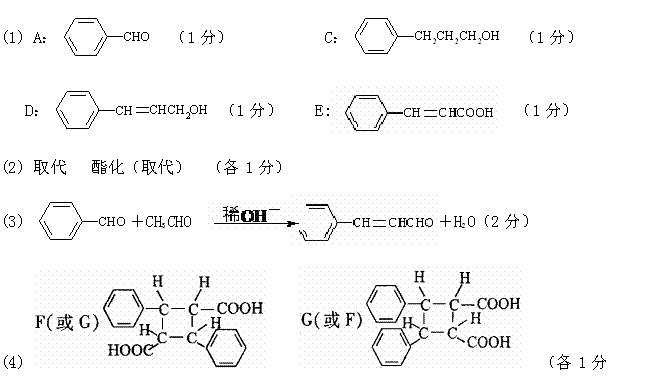
��1��д���л���Ľṹ��ʽ�� A____ __��C____ ____��D______ ___��E ��
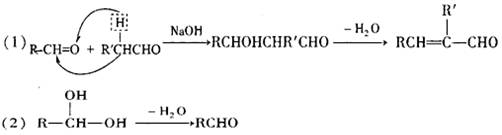
��2����Ӧ��I����������________ ______����Ӧ��II����������______ ________��
��3����ɻ�ѧ����ʽ: A��B��___________________ __________________��
��4��ͬ���ࡢ��Ϊͬ���칹��� F �� G �Ľṹ��ʽΪ��
F__________________________��G__________ _______________��
��ϰ��ϵ�д�
�����Ŀ

 2NH3����һ���¶��£���2L�ܱ������У�����2molN2��5molH2��һ��������ʹ֮��Ӧ������2min��ﵽƽ��״̬�����NH3Ϊ0.4mol����
2NH3����һ���¶��£���2L�ܱ������У�����2molN2��5molH2��һ��������ʹ֮��Ӧ������2min��ﵽƽ��״̬�����NH3Ϊ0.4mol����
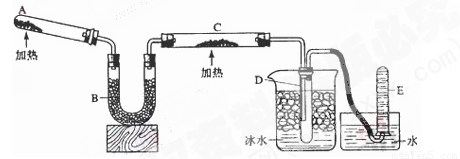
 N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ���ش��������⣺
N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ���ش��������⣺