��Ŀ����
��21�֣���1����50mL 0.55mol/L NaOH��Һ��50mL 0.25mol/L H2SO4��Һ�����к��Ȳⶨ��ʵ�飬�����Һ�ڷ�Ӧǰ����¶ȱ仯Ϊt1�桫t2�棨t2>t1��,��Ϻ���Һ�ı�����Ϊc = 4.18J����g���棩,��Һ���ܶȶ�����Ϊ1g/mL���к��ȡ�H=__________(�����ʽ�����û���)������H2SO4��Һ������ͬ���ʵ���Ũ�ȵ�CH3COOH��Һ����õġ�H______��(��ƫ����ƫС������ͬ����������ϡ���ỻ��Ũ����������ʵ�飬��õġ�H_______���ƫ����ƫС������ͬ������
��2��ij���ܻ�ѧ����N2H2�ڣ���Ԫ�ص��ӻ�����Ϊ____������ʽΪ____��һ���������ЦҼ� �� ���� �� ����
��3�������ܱ������н��п��淴Ӧ�� CO(g)��NO2(g) CO2(g)��NO(g)��(����Ӧ����)���ﵽƽ���ֻ�ı�����һ����������ƽ���Ӱ���ǣ�
CO2(g)��NO(g)��(����Ӧ����)���ﵽƽ���ֻ�ı�����һ����������ƽ���Ӱ���ǣ�
�����������������ƽ�������ƶ�(�������������)����Ӧ��������ɫ��������(��������dz�������䡱)
������������䣺��ͨ��CO2���壬ƽ���� ���ƶ�����Ӧ��������ɫ��������ͨ��N2���壬ƽ���� ���ƶ�����Ӧ��������ɫ�������ۼ��������ƽ���������ƶ���
(4)�±��Ǽ��ֳ���ȼ��(1 mol)��ȫȼ��ʱ�ų���������
| ���� | ̿��(C) | һ����̼(CO) | ����(H2) | ����(CH4) | �Ҵ�(C2H5OH) |
| ״̬ | ���� | ���� | ���� | ���� | Һ�� |
| ����(kJ) | 392.8 | 282.6 | 285.8 | 890.3 | 1 367 |
��д���ܵ�ú���е�һ����̼ȼ���ȵ��Ȼ�ѧ����ʽ_______________________
�۳��ȼ��1 mol���и���ȼ�ϣ��ŷų�������̼����������________��
�ܿ���ȼ�ϴ������ޣ�������ȼ�չ����л������Ⱦ��������Դ�������ķ�չս�ԣ��ҹ��������õ���ɫ��Դ��________�ȡ�
��21�֣���1��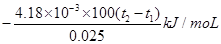 ƫ�� ƫС����2�֣�
ƫ�� ƫС����2�֣�
��2��sp2�ӻ� 3 1 ����1�֣�
��3��������dz ���� ���� �� ���� ����1�֣�
��4��(1)���飨1�֣� (2)CO(g)��1/2O2(g)===CO2(g) ��H����282.6 kJ��mol��1
��2�֣�
(3)�Ҵ���1�֣� (4)���ܡ�̫����(����ܡ�������)��1�֣�
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���21�֣���1����50mL 0.55mol/L NaOH��Һ��50mL 0.25mol/LH2SO4��Һ�����к��Ȳⶨ��ʵ�飬�����Һ�ڷ�Ӧǰ����¶ȱ仯Ϊt1�桫t2�� ��t2>t1��,��Ϻ���Һ�ı�����Ϊc = 4.18J����g���棩,��Һ���ܶȶ�����Ϊ1g/mL���к��ȡ�H=__________(�����ʽ�����û���)������H2SO4��Һ������ͬ���ʵ���Ũ�ȵ�CH3COOH��Һ����õġ�H______��(��ƫ����ƫС������ͬ����������ϡ���ỻ��Ũ����������ʵ�飬��õġ�H_______���ƫ����ƫС������ͬ������
��2��ij���ܻ�ѧ����N2H2�ڣ���Ԫ�ص��ӻ�����Ϊ____������ʽΪ____��һ���������ЦҼ� �� ���� �� ����
��3�������ܱ������н��п��淴Ӧ�� CO(g)��NO2(g)CO2(g)��NO(g)��(����Ӧ����)���ﵽƽ���ֻ�ı�����һ����������ƽ���Ӱ���ǣ�
�����������������ƽ���� �� �ƶ�(�������������)����Ӧ��������ɫ��������(��������dz�������䡱)
������������䣺��ͨ��CO2���壬ƽ���� ���ƶ�����Ӧ��������ɫ�� �� ����ͨ��N2���壬ƽ���� ���ƶ�����Ӧ��������ɫ�� �� ���ۼ��������ƽ���������ƶ���
(4)�±��Ǽ��ֳ���ȼ��(1 mol)��ȫȼ��ʱ�ų���������
| ���� | ̿��(C) | һ����̼(CO) | ����(H2) | ����(CH4) | �Ҵ�(C2H5OH) |
| ״̬ | ���� | ���� | ���� | ���� | Һ�� |
| ����(kJ) | 392.8 | 282.6 | 285.8 | 890.3 | 1 367 |
�ٴ������Ƕȷ�����Ŀǰ���ʺϼ�ͥʹ�õ���������ȼ����________��
��д���ܵ�ú���е�һ����̼ȼ���ȵ��Ȼ�ѧ����ʽ_______________________
�۳��ȼ��1mol���и���ȼ�ϣ��ŷų�������̼����������________��
�ܿ���ȼ�ϴ������ޣ�������ȼ�չ����л������Ⱦ��������Դ�������ķ�չս�ԣ��ҹ��������õ���ɫ��Դ��________�ȡ�
��21�֣���1����50mL 0.55mol/L NaOH��Һ��50mL 0.25mol/L H2SO4��Һ�����к��Ȳⶨ��ʵ�飬�����Һ�ڷ�Ӧǰ����¶ȱ仯Ϊt1�桫t2�� ��t2>t1��,��Ϻ���Һ�ı�����Ϊc = 4.18J����g���棩,��Һ���ܶȶ�����Ϊ1g/mL���к��ȡ�H=__________(�����ʽ�����û���)������H2SO4��Һ������ͬ���ʵ���Ũ�ȵ�CH3COOH��Һ����õġ�H______��(��ƫ����ƫС������ͬ����������ϡ���ỻ��Ũ����������ʵ�飬��õġ�H_______���ƫ����ƫС������ͬ������
��2��ij���ܻ�ѧ����N2H2�ڣ���Ԫ�ص��ӻ�����Ϊ____������ʽΪ____��һ���������ЦҼ� �� ���� �� ����
��3�������ܱ������н��п��淴Ӧ�� CO(g)��NO2(g) CO2(g)��NO(g)��(����Ӧ����)���ﵽƽ���ֻ�ı�����һ����������ƽ���Ӱ���ǣ�
CO2(g)��NO(g)��(����Ӧ����)���ﵽƽ���ֻ�ı�����һ����������ƽ���Ӱ���ǣ�

�����������������ƽ���� �� �ƶ�(�������������)����Ӧ��������ɫ��������(��������dz�������䡱)
������������䣺��ͨ��CO2���壬ƽ���� ���ƶ�����Ӧ��������ɫ�� �� ����ͨ��N2���壬ƽ���� ���ƶ�����Ӧ��������ɫ�� �� ���ۼ��������ƽ���������ƶ���
(4)�±��Ǽ��ֳ���ȼ��(1 mol)��ȫȼ��ʱ�ų���������
|
���� |
̿��(C) |
һ����̼(CO) |
����(H2) |
����(CH4) |
�Ҵ�(C2H5OH) |
|
״̬ |
���� |
���� |
���� |
���� |
Һ�� |
|
����(kJ) |
392.8 |
282.6 |
285.8 |
890.3 |
1 367 |
�ٴ������Ƕȷ�����Ŀǰ���ʺϼ�ͥʹ�õ���������ȼ����________��
��д���ܵ�ú���е�һ����̼ȼ���ȵ��Ȼ�ѧ����ʽ_______________________
�۳��ȼ��1 mol���и���ȼ�ϣ��ŷų�������̼����������________��
�ܿ���ȼ�ϴ������ޣ�������ȼ�չ����л������Ⱦ��������Դ�������ķ�չս�ԣ��ҹ��������õ���ɫ��Դ��________�ȡ�

