��Ŀ����
��12�֣�ѧϰ±��������ʱ������֪���������ڲ�ͬ�ܼ������������Ʒ�����ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ������ǿ���ͨ��ʵ��ķ���ȥ��֤��Ӧ�IJ��
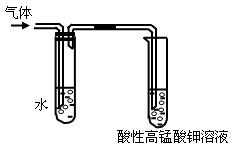
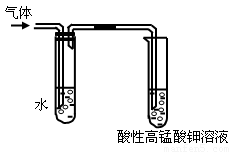
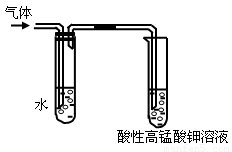
��1�������������������Ƶ��Ҵ���Һ�� ����Ӧ���ͣ��п��Թ۲쵽���������ɡ�ijͬѧ������ͼ��ʾװ�öԸ����������֤�������۲쵽�������� ��
��2��ʢˮ�Թܵ������� ��
��3�����������Ը��������Һ�⣬�������� �Լ���������壬�䷴Ӧԭ���� ���û�ѧ����ʽ��ʾ����
��4����һλͬѧȡ��������������������ˮ��Һ��Ӧ��Ļ����Һ�������еμ���������Һ�����ȣ�����������������ͬѧ�ɴ˵ó�����������������ˮ��Һ��Ӧ���������廯�ơ���ʦ��ͬѧ����Ϊ��������������������ʵ����֤�÷�Ӧ���������廯�ƣ������ͬѧʵ�鷽����ͬ��ʵ�鷽���� ��
��12�֣���1�� ��ȥ��Ӧ ���������ɫ
��2�� ��ȥ�������������Ҵ�
��3����ˮ CH2==CH2 + Br2 �� BrCH2CH2Br
��4���������Һ����ϡ�����ữ�������������м���������Һ
����:

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�