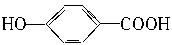��Ŀ����
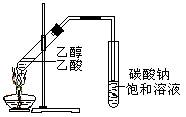
��11�֣������dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
��1��д����ȡ���������Ļ�ѧ��Ӧ����ʽ����ָ���䷴Ӧ���ͣ�_____��_________����������_______________��Ӧ��
��2��Ũ����������ǣ���_________________________����____________________��
��3��װ����ͨ�����ĵ���Ҫ����_____________��Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹ_______����
��4����Ҫ���Ƶõ������������������Ӧ���õ�ʵ�������___________��
A������ B����Һ C������ D���ᾧ
��5����30��������46���Ҵ���Ӧ�����ʵ�ʲ��������۲��ʵ�67%����ɵõ�����������������______________��
A��29.5�� B��44�� C��74.8�� D��88��

��1��д����ȡ���������Ļ�ѧ��Ӧ����ʽ����ָ���䷴Ӧ���ͣ�_____��_________����������_______________��Ӧ��
��2��Ũ����������ǣ���_________________________����____________________��
��3��װ����ͨ�����ĵ���Ҫ����_____________��Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹ_______����
��4����Ҫ���Ƶõ������������������Ӧ���õ�ʵ�������___________��
A������ B����Һ C������ D���ᾧ
��5����30��������46���Ҵ���Ӧ�����ʵ�ʲ��������۲��ʵ�67%����ɵõ�����������������______________��
A��29.5�� B��44�� C��74.8�� D��88��
��1��CH3COOH+CH3CH2OH
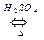 CH3COOCH2CH3+H2O ��2�֣�ȡ������������1�֣�
CH3COOCH2CH3+H2O ��2�֣�ȡ������������1�֣���2���ٴ�����1�֣�������ˮ����1�֣�
��3������Na2CO3��1�֣���������1�֣�
��4��B��2�֣�
��5��A��2�֣�
��

��ϰ��ϵ�д�
 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
�����Ŀ