��Ŀ����
ȼ�Ϻ���Դ�ǻ�ѧ֪ʶ�����������ϵ��Ϊ���е����ݣ�����Ҫ��ע������Դ�ĺ������ã������о�����������Դ����1������ԴӦ�þ���ԭ״���á�ȼ��ʱ�������������Ҳ�����Ⱦ�����ص㣮��ú̿��ʯ�͡�ú���������У�ǰ;��������Դ��______��
��2�����������ҹ�ú���¹ʴ����������˹��ը���£���˹�к��м����һ����̼�����壬��������˹Ũ�ȴﵽһһ����Χʱ������ȼ�ձ�ը��Ϊ�������ѵķ���Ӧ��ȡ����ʵ���еĴ�ʩ��______������ţ�
�ټ�ǿ��ȫ�������ž�����Դ
�ڽ�����˹������Ż��
�����ͨ�������ܽ����е�������ȥ
��3��Ϊ�����ú����ЧӦ��ͬʱ����ȼ��ʱ�Ļ�����Ⱦ������úת��Ϊˮú�������ǽ�úת��Ϊ�ྻȼ�ϵķ���֮һ��ˮú������Ҫ�ɷ���һ����̼��������������ú̿��ˮ������Ӧ�Ƶã���֪C��ʯī����CO��H2ȼ�յ��Ȼ�ѧ����ʽΪ��
C��s��ʯī��+O2��g���TCO2��g������H1=-393.5kJ?mol-1
H2��g��+
 O2��g���TH2O��g������H2=-241.8kJ?mol-1
O2��g���TH2O��g������H2=-241.8kJ?mol-1CO��g��+O
 2��g���TCO2��g������H3=-283.0kJ?mol-1
2��g���TCO2��g������H3=-283.0kJ?mol-1H2��g��+
 O2��g���TH2O��l������H4=-285.8kJ?mol-1
O2��g���TH2O��l������H4=-285.8kJ?mol-1��ش��������⣺
�ٸ��������ṩ���Ȼ�ѧ����ʽ���㣬36gˮ��Һ̬�����̬�������仯��______��
��д��C��s��ʯī����ˮ������Ӧ���Ȼ�ѧ����ʽ______��
�۱�����Һ��ʯ��������Ҫ�ɷ�֮һ������ȼ�յ��Ȼ�ѧ����ʽΪ��
C3H8��g��+5O2��g���T3CO2��g��+4H2O��g������H=-2220.0kJ?mol-1
��ͬ���ʵ����ı����һ����̼��ȫȼ��������̬����ʱ������������֮��Ϊ______����ͬ�����������ͱ�����ȫȼ��������̬����ʱ������������֮��Ϊ______��
���𰸡���������1��ú̿��ʯ�����ڻ�ʯ��Դ��ȼ��������Ⱦ������ú��ȼ�ջ����ɵ�������ЧӦ�����壬����ȼ������ˮ����ֵ�ߡ�����Ⱦ��
��2���ž�����Դ�����ͨ���������Է�ֹ��˹��ը����ȼ����Ż��һ������²��ܸı䣻�����е������龡�Dz���ʵ�ģ�Ҳ�Dz����Եģ�
��3����������ȼ������H2O��g��������H2O��l�����Ȼ�ѧ����ʽ��֪��H2O��l��=H2O��g����H=+44kJ/mol���ݴ˼��㣻
�ڸ��ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ�����ʵ���ϵ�����мӼ�����Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ��ϵ��������Ӧ�ļ��㣻
�۸����Ȼ�ѧ����ʽ������ͬ���ʵ����ı����һ����̼��ȫȼ��������̬����ʱ����������֮�ȣ�
������Ϊ1g������n=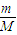 �������ʵ������ٸ����Ȼ�ѧ����ʽ���㣮
�������ʵ������ٸ����Ȼ�ѧ����ʽ���㣮
����⣺��1��ú̿��ʯ�����ڻ�ʯ��Դ��ȼ��������Ⱦ������ú��ȼ�ջ����ɵ�������ЧӦ�����壬����ȼ������ˮ����ֵ�ߡ�����Ⱦ����ǰ;��������Դ��
�ʴ�Ϊ��������
��2���ټ�ǿ��ȫ�������ž�����Դ���Է�ֹ��˹��ը���ʢ���ȷ��
����˹���Ż��һ������²��ܸı䣬�ʢڴ���
�����ͨ���������Խ�����˹��Ũ�ȣ����Է�ֹ��˹��ը���ʢ���ȷ��
�ܽ����е������龡�Dz���ʵ�ģ�Ҳ�Dz������ģ���Ϊ�����ھ�����ҵ��Ҫ�������ʢܴ���
�ʴ�Ϊ���٢ۣ�
��3������֪��H2��g��+ O2��g���TH2O��g������H2=-241.8kJ?mol-1
O2��g���TH2O��g������H2=-241.8kJ?mol-1
H2��g��+ O2��g���TH2O��l������H4=-285.8kJ?mol-1
O2��g���TH2O��l������H4=-285.8kJ?mol-1
���ݸ�˹���ɿɵã�H2O��l��=H2O��g����H=+44kJ/mol��
��36gˮ��Һ̬�����̬��Ӧ��������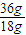 ×44kJ=88kJ��
×44kJ=88kJ��
�ʴ�Ϊ������88kJ��
����֪����C��s��ʯī��+O2��g���TCO2��g������H1=-393.5kJ?mol-1
��H2��g��+ O2��g���TH2O��g������H2=-241.8kJ?mol-1
O2��g���TH2O��g������H2=-241.8kJ?mol-1
��CO��g��+ O2��g���TCO2��g������H3=-283.0kJ?mol-1
O2��g���TCO2��g������H3=-283.0kJ?mol-1
���ݸ�˹���ɣ���-��-��ɵ�C��s��ʯī��+H2O��g��=CO��g��+H2��g����H=+131.3kJ?mol-1��
�ʴ�Ϊ��C��s��ʯī��+H2O��g��=CO��g��+H2��g����H=+131.3kJ?mol-1��
�۸����Ȼ�ѧ����ʽ��֪����ͬ���ʵ����ı����һ����̼��ȫȼ��������̬����ʱ����������֮��Ϊ2220.0kJ?mol-1��283.0kJ?mol-1=2220��283
������Ϊ1g�����������ʵ���Ϊ0.5mol���������ʵ���Ϊ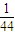 mol���ʷų�������֮��Ϊ0.5mol×241.8kJ?mol-1��
mol���ʷų�������֮��Ϊ0.5mol×241.8kJ?mol-1�� mol×2220.0kJ?mol-1=5319.6��2220��
mol×2220.0kJ?mol-1=5319.6��2220��
�ʴ�Ϊ��2220��283��5319.6��2220��
���������⿼���Ȼ�ѧ����ʽ����д���йؼ��㣬�Ѷ��еȣ�ע�������Ȼ�ѧ����ʽ���������˹���ɣ�
��2���ž�����Դ�����ͨ���������Է�ֹ��˹��ը����ȼ����Ż��һ������²��ܸı䣻�����е������龡�Dz���ʵ�ģ�Ҳ�Dz����Եģ�
��3����������ȼ������H2O��g��������H2O��l�����Ȼ�ѧ����ʽ��֪��H2O��l��=H2O��g����H=+44kJ/mol���ݴ˼��㣻
�ڸ��ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ�����ʵ���ϵ�����мӼ�����Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ��ϵ��������Ӧ�ļ��㣻
�۸����Ȼ�ѧ����ʽ������ͬ���ʵ����ı����һ����̼��ȫȼ��������̬����ʱ����������֮�ȣ�
������Ϊ1g������n=
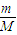 �������ʵ������ٸ����Ȼ�ѧ����ʽ���㣮
�������ʵ������ٸ����Ȼ�ѧ����ʽ���㣮����⣺��1��ú̿��ʯ�����ڻ�ʯ��Դ��ȼ��������Ⱦ������ú��ȼ�ջ����ɵ�������ЧӦ�����壬����ȼ������ˮ����ֵ�ߡ�����Ⱦ����ǰ;��������Դ��
�ʴ�Ϊ��������
��2���ټ�ǿ��ȫ�������ž�����Դ���Է�ֹ��˹��ը���ʢ���ȷ��
����˹���Ż��һ������²��ܸı䣬�ʢڴ���
�����ͨ���������Խ�����˹��Ũ�ȣ����Է�ֹ��˹��ը���ʢ���ȷ��
�ܽ����е������龡�Dz���ʵ�ģ�Ҳ�Dz������ģ���Ϊ�����ھ�����ҵ��Ҫ�������ʢܴ���
�ʴ�Ϊ���٢ۣ�
��3������֪��H2��g��+
 O2��g���TH2O��g������H2=-241.8kJ?mol-1
O2��g���TH2O��g������H2=-241.8kJ?mol-1H2��g��+
 O2��g���TH2O��l������H4=-285.8kJ?mol-1
O2��g���TH2O��l������H4=-285.8kJ?mol-1���ݸ�˹���ɿɵã�H2O��l��=H2O��g����H=+44kJ/mol��
��36gˮ��Һ̬�����̬��Ӧ��������
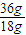 ×44kJ=88kJ��
×44kJ=88kJ���ʴ�Ϊ������88kJ��
����֪����C��s��ʯī��+O2��g���TCO2��g������H1=-393.5kJ?mol-1
��H2��g��+
 O2��g���TH2O��g������H2=-241.8kJ?mol-1
O2��g���TH2O��g������H2=-241.8kJ?mol-1��CO��g��+
 O2��g���TCO2��g������H3=-283.0kJ?mol-1
O2��g���TCO2��g������H3=-283.0kJ?mol-1���ݸ�˹���ɣ���-��-��ɵ�C��s��ʯī��+H2O��g��=CO��g��+H2��g����H=+131.3kJ?mol-1��
�ʴ�Ϊ��C��s��ʯī��+H2O��g��=CO��g��+H2��g����H=+131.3kJ?mol-1��
�۸����Ȼ�ѧ����ʽ��֪����ͬ���ʵ����ı����һ����̼��ȫȼ��������̬����ʱ����������֮��Ϊ2220.0kJ?mol-1��283.0kJ?mol-1=2220��283
������Ϊ1g�����������ʵ���Ϊ0.5mol���������ʵ���Ϊ
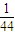 mol���ʷų�������֮��Ϊ0.5mol×241.8kJ?mol-1��
mol���ʷų�������֮��Ϊ0.5mol×241.8kJ?mol-1�� mol×2220.0kJ?mol-1=5319.6��2220��
mol×2220.0kJ?mol-1=5319.6��2220���ʴ�Ϊ��2220��283��5319.6��2220��
���������⿼���Ȼ�ѧ����ʽ����д���йؼ��㣬�Ѷ��еȣ�ע�������Ȼ�ѧ����ʽ���������˹���ɣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 O2��g��==H2O��g����H2=��241��8kJ��mol-1
O2��g��==H2O��g����H2=��241��8kJ��mol-1  O2��g��==CO2��g����H3=��283��0kJ��mol-1
O2��g��==CO2��g����H3=��283��0kJ��mol-1