��Ŀ����
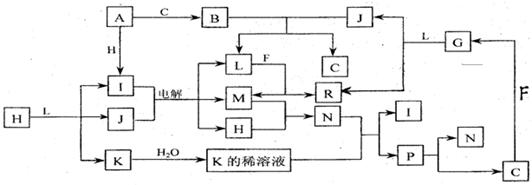
��֪ : ���и������ʶ��ɶ�����Ԫ����� , ����֮��Ĺ�ϵ����ͼ��ʾ��
������ ,A �� F Ϊ�������� , F ������ L ��Һ��Ӧ, ������ N ��Һ��Ӧ�� C�� H �� M Ϊ���嵥�� , ���� H �ʻ���ɫ ,A �� B �� I �� K �� L �� R ����ɫ��Ӧ���ʻ�ɫ����ش� :
(l)P�ĽṹʽΪ ��B �ĵ���ʽΪ___________________��
(2)Rˮ��Һ�ʼ��Ե�ԭ��������ӷ���ʽ��ʾΪ ��
(3)����һ����������B��Ӧ����C.д�������Ӧ�Ļ�ѧ����ʽ_ ��
(4)����һ����������J��Ӧ����C,д�������Ӧ�Ļ�ѧ����ʽ_ ��
(5)ij����Q����Ư������,��Q��H�����ʵ���ͨ��ˮ��������Һû��Ư������ ,д�����������ӷ�Ӧ����ʽ ��
������ ,A �� F Ϊ�������� , F ������ L ��Һ��Ӧ, ������ N ��Һ��Ӧ�� C�� H �� M Ϊ���嵥�� , ���� H �ʻ���ɫ ,A �� B �� I �� K �� L �� R ����ɫ��Ӧ���ʻ�ɫ����ش� :

(l)P�ĽṹʽΪ ��B �ĵ���ʽΪ___________________��
(2)Rˮ��Һ�ʼ��Ե�ԭ��������ӷ���ʽ��ʾΪ ��
(3)����һ����������B��Ӧ����C.д�������Ӧ�Ļ�ѧ����ʽ_ ��
(4)����һ����������J��Ӧ����C,д�������Ӧ�Ļ�ѧ����ʽ_ ��
(5)ij����Q����Ư������,��Q��H�����ʵ���ͨ��ˮ��������Һû��Ư������ ,д�����������ӷ�Ӧ����ʽ ��
(1) H��O��C�죬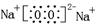
�� (2) AlO2- + 2H2O ? Al(OH)3 + OH-
(3)2C02 + 2Na202 = 2Na2C03 + O2
(4)2F2 + 2H20 =" 4HF" + O2
(5)S02 + Cl2 + 2H20 = 4H+ + 2Cl- + SO42
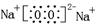
�� (2) AlO2- + 2H2O ? Al(OH)3 + OH-
(3)2C02 + 2Na202 = 2Na2C03 + O2
(4)2F2 + 2H20 =" 4HF" + O2
(5)S02 + Cl2 + 2H20 = 4H+ + 2Cl- + SO42
��

��ϰ��ϵ�д�
�����Ŀ
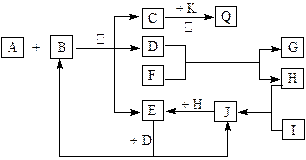
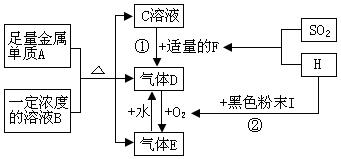
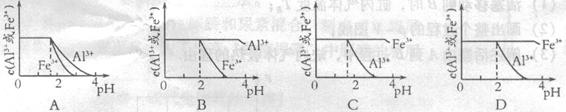
 mol4��Lһ4������pH=0��Ũ�Ⱦ�Ϊ0��04mol��L-1��Al3+��Fe3+��Һ�м���A������������Ӧˮ�������Һ���Ե���pH(����Һ�������)���ù�����Al3+��Fe3+��Ũ����pH��ϵ��ȷ����
mol4��Lһ4������pH=0��Ũ�Ⱦ�Ϊ0��04mol��L-1��Al3+��Fe3+��Һ�м���A������������Ӧˮ�������Һ���Ե���pH(����Һ�������)���ù�����Al3+��Fe3+��Ũ����pH��ϵ��ȷ����