��Ŀ����
п��һ�ֳ��ý������أ�Ga���Ļ����ﵪ���أ�GaN��������LED����Ҫ���ϣ�����Ϊ�������뵼����ϡ�
I���أ�Ga���ǻ�ұ��п�����еĸ���Ʒ��������ͬ���������ڣ���ѧ�����������ơ�
��1��Ga��ԭ�ӽṹʾ��ͼΪ___________________��
��2�� GaN����Ga��NH3�ڸ�����������ȡ���÷�Ӧ�Ļ�ѧ����ʽ_______________��
��3�������й��غ��صĻ������˵����ȷ����____________
A�������£�Ga����ˮ���ҷ�Ӧ�ų�����
B��һ�������£�Ga�������������������
C��һ�������£�Ga2O3����NaOH��Ӧ������
D��Ga2O3����Ga(OH)3���ȷֽ�õ�
II��п��ұ�������л�ʪ������ҵ������п��ɰ����Ҫ��Zn0��ZnFe2O4������������CaO��FeO��CuO��NiO�������ʪ����ȡ����п��������ͼ��ʾ��

��֪��Fe�Ļ�����ǿ��Ni
��4��ZnFe2O4����д��ZnO��Fe2O3��ZnFe2O4��H2SO4��Ӧ�Ļ�ѧ����ʽΪ____ _��
��5������I������Ϊ��������һ���ǽ���Һ��������Fe2+�������ڶ����ǿ�����ҺpH��ֻʹFe3+ת��ΪFe(OH)3����������I���ɵij����л�������Һ�е��������ʣ���Һ�е��������ʱ���ͬ������ԭ����________________________��
��6������II�м���Zn��Ŀ����_______________________��
��7�������£�����I�У����Ҫʹc(Fe3+) < 10-5 mol/L����Ӧ������ҺpH�ķ�ΧΪ_____________����֪��Ksp[Fe(OH)3]=8.0��10-38��lg5=0.7
��ѧ����ᡢ����������ء��������������ʵ�Ľ�����ȷ����
ѡ�� | �������ʵ | ���� |
A | ���ȵ��ռ���Һϴȥ���� | Na2CO3��ֱ�Ӻ����۷�Ӧ |
B | Ư���ڿ����о��ñ��� | Ư���е�CaCl2 ������е�CO2��Ӧ����CaCO3 |
C | ʩ��ʱ����ľ��(��Ч�ɷ�ΪK2CO3)������NH4Cl���ʹ�� | K2CO3��NH4Cl��Ӧ���ɰ����ή�ͷ�Ч |
D | FeCl3��Һ������ͭ��ӡˢ��·������ | FeCl3�ܴӺ���Cu2������Һ���û���ͭ |


 O��NaOH�Ļ����Һ��Na+��SO42�C��Cl�C��ClO�C��OH�C
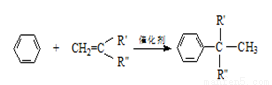
O��NaOH�Ļ����Һ��Na+��SO42�C��Cl�C��ClO�C��OH�C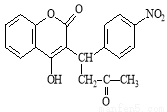

 + H2O
+ H2O