��Ŀ����
���к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȡ������²��裺
��1�����ƴ���Һ����5.0 g�����������ʣ����ʲ������ᷴӦ���Ĺ����ռ���Ʒ����1 L��Һ��
L��Һ��
��2�����
��ʢװ0.100 0 mol��L-1�����ҺӦ��ʹ�õĵζ����� ����ס����ҡ�����
������ƿ�м���һ������Ĵ�����Һ��������ָʾ����
�۵ζ��������۾�Ӧ ��
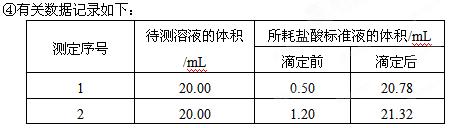
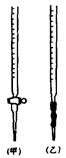
��3�����㴿�ȣ��ռ���Ʒ�Ĵ�����_________________��
��4�������������в�����ʹ�ⶨ���ƫ����� �����ţ�3
��������ˮ��ϴ��ƿ��
���ڵζ������в�����������Һ������ƿ�⣻
�۶�ȡ�ζ����յ����ʱ�����Ӷ�����
�ܵζ�ǰ�ζ��ܼ��������ݣ��ζ������������ʧ��
��ȡ����Һ�ĵζ���������ˮϴ��δ��ϴ��
��1�����ƴ���Һ����5.0 g�����������ʣ����ʲ������ᷴӦ���Ĺ����ռ���Ʒ����1
 L��Һ��
L��Һ����2���ζ���
��ʢװ0.100 0 mol��L-1�����ҺӦ��ʹ�õĵζ����� ����ס����ҡ�����
������ƿ�м���һ������Ĵ�����Һ��������ָʾ����
�۵ζ��������۾�Ӧ ��


��3�����㴿�ȣ��ռ���Ʒ�Ĵ�����_________________��
��4�������������в�����ʹ�ⶨ���ƫ����� �����ţ�3
��������ˮ��ϴ��ƿ��
���ڵζ������в�����������Һ������ƿ�⣻
�۶�ȡ�ζ����յ����ʱ�����Ӷ�����
�ܵζ�ǰ�ζ��ܼ��������ݣ��ζ������������ʧ��
��ȡ����Һ�ĵζ���������ˮϴ��δ��ϴ��
��1���� ��2����ƿ����Һ��ɫ�ı仯 ��3��80.8% ��4���ڢ�
��2����ƿ����Һ��ɫ�ı仯 ��3��80.8% ��4���ڢ�
 ��2����ƿ����Һ��ɫ�ı仯 ��3��80.8% ��4���ڢ�
��2����ƿ����Һ��ɫ�ı仯 ��3��80.8% ��4���ڢ���

��ϰ��ϵ�д�
�����Ŀ

 ����ţ�
����ţ� ��ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽�____________(����ڡ����ڡ�)�ұߵ����̣���ʹ��ƽƽ�⣬�����еIJ���Ϊ____________���ٶ����ճ���С�ձ�������Ϊ________(�32.6 g����32.61 g��)��
��ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽�____________(����ڡ����ڡ�)�ұߵ����̣���ʹ��ƽƽ�⣬�����еIJ���Ϊ____________���ٶ����ճ���С�ձ�������Ϊ________(�32.6 g����32.61 g��)��
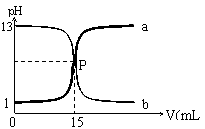
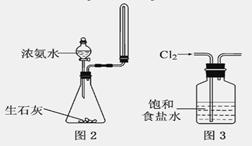
 ����������ֵ�������Ӧ����ֹͣ����
����������ֵ�������Ӧ����ֹͣ���� Cl2�е�����HCl
Cl2�����HCl