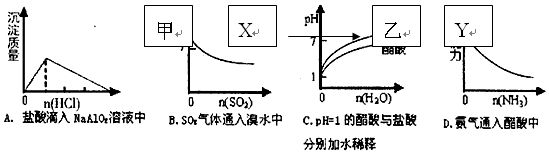
��Ŀ����
��16�֣������ԭ��������Na 23 O 16 H 1��
��1���������ʵ���Ũ��Ϊ0.2 mol/L��NaOH��Һ500 mL����ղ���ش��������⣺
��2�����в��������Ƶ�NaOH��ҺŨ����ƫ��Ӱ�����_______
��3����0.1 mol/L��AlCl3��Һ�м��������0.2 mol/L��NaOH��Һ��������Ӧ�����ӷ���ʽΪ
��4�������ƺõ�0.2 mol/L��NaOH��Һ��μ��뵽0.1 mol/L��Ca��HCO3��2��Һ�У�������Ӧ�����ӷ���ʽΪ
��1���������ʵ���Ũ��Ϊ0.2 mol/L��NaOH��Һ500 mL����ղ���ش��������⣺
| Ӧ����NaOH������/g | �Ѹ����� | ���Ѹ��������Ҫ���������� |
| | �ձ���ҩ�ס� ������ƽ | |
| A������NaOH����ʱ��¶���ڿ�����ʱ����� |
| B��ѡ�õ�����ƿ��������������ˮ |
| C�����ձ����ܽ�NaOH��������������Һע������ƿ�� |
| D���ڶ���ʱ��������ƿ�̶��� |
��4�������ƺõ�0.2 mol/L��NaOH��Һ��μ��뵽0.1 mol/L��Ca��HCO3��2��Һ�У�������Ӧ�����ӷ���ʽΪ
��16�֣�
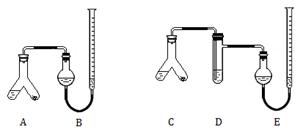
��1��4.0��2�֣�����4�ĸ�1�֣���500 mL����ƿ������������ͷ�ιܡ���Ͳ��8�֣�������ƿ��д����֣�д�����ֲ����֣�
��2��C��2�֣�
��3��Al3+ + 4OH�� = AlO2�� + 2H2O��2�֣�
��4��Ca2+ + HCO3�� + OH�� = CaCO3��+ H2O��2�֣�
��1��4.0��2�֣�����4�ĸ�1�֣���500 mL����ƿ������������ͷ�ιܡ���Ͳ��8�֣�������ƿ��д����֣�д�����ֲ����֣�
��2��C��2�֣�
��3��Al3+ + 4OH�� = AlO2�� + 2H2O��2�֣�
��4��Ca2+ + HCO3�� + OH�� = CaCO3��+ H2O��2�֣�
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ