��Ŀ����
��12�֣�������Ԫ��A��B��C��D��E��ԭ����������������Ԫ��������Ϣ���±���
(1)E�ļ����ӵĽṹʾ��ͼΪ____________��
(2) D�����ڱ��е�λ��Ϊ ________________��
(3)B��C�����ӵİ뾶��СΪ________ __��(�����ӷ�
__��(�����ӷ� �ź͡�>������=����<����ʾ)
�ź͡�>������=����<����ʾ)
(4)Ԫ�طǽ�����ǿ���Ƚ��кܶ��������B��E�ķǽ�����ǿ�����о������в����е���_________������ţ���
a.�Ƚ����ֵ��ʵ���ɫ
b.�Ƚ��⻯����ȶ���
c.������Ԫ�������ڱ��е�λ��
d.������Ԫ�ص�������Ȼ���еĴ���״̬
(5)BԪ�ؾ�������������Ԫ���е�һ���γɻ��������ֻ�����Ӽ�����___________�� д��ѧʽ����ͬ�����Ⱥ����Ӽ��ֺ����ۼ�����______________��
д��ѧʽ����ͬ�����Ⱥ����Ӽ��ֺ����ۼ�����______________��
| Ԫ�ر�� | Ԫ��������Ϣ |
| A | ������ۺ�����۵ľ���ֵ֮��Ϊ2 |
| B | �������������ڲ��������3�� |
| C | 1molC�������� ��ˮ��Ӧ���ڱ�״��������11.2LH2 ��ˮ��Ӧ���ڱ�״��������11.2LH2 |
| D | ԭ�������������������������� |
| E | �����������ӵĵ��Ӳ�ṹ��Arԭ����ͬ |
(2) D�����ڱ��е�λ��Ϊ ________________��
(3)B��C�����ӵİ뾶��СΪ________
 __��(�����ӷ�
__��(�����ӷ� �ź͡�>������=����<����ʾ)
�ź͡�>������=����<����ʾ)(4)Ԫ�طǽ�����ǿ���Ƚ��кܶ��������B��E�ķǽ�����ǿ�����о������в����е���_________������ţ���
a.�Ƚ����ֵ��ʵ���ɫ
b.�Ƚ��⻯����ȶ���
c.������Ԫ�������ڱ��е�λ��
d.������Ԫ�ص�������Ȼ���еĴ���״̬
(5)BԪ�ؾ�������������Ԫ���е�һ���γɻ��������ֻ�����Ӽ�����___________��
 д��ѧʽ����ͬ�����Ⱥ����Ӽ��ֺ����ۼ�����______________��
д��ѧʽ����ͬ�����Ⱥ����Ӽ��ֺ����ۼ�����______________����1��
��2����������IIIA��
��3��O2-��Na+
��4��ad
��5��Na2O��Al2O3��Na2O2

��2����������IIIA��
��3��O2-��Na+
��4��ad
��5��Na2O��Al2O3��Na2O2
��

��ϰ��ϵ�д�
�����Ŀ
 ������
������ ��
�� �·�Ӧ��
�·�Ӧ��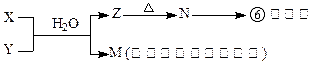
 ����ˮ����ˮ��Һ�е����X����Z����XZ��ˮ��Һ��ʹʯ����Һ��졣
����ˮ����ˮ��Һ�е����X����Z����XZ��ˮ��Һ��ʹʯ����Һ��졣 ��D218O���й�˵����ȷ���� �� ��
��D218O���й�˵����ȷ���� �� ��