��Ŀ����
T��X��Y��Z��Q��R��P��WΪ���ڱ�ǰ������Ԫ�أ�ԭ���������ε������������Ϣ�����
��1��X��Y��Z����Ԫ�صĵ�һ�������ɴ�С��˳����______����Ԫ�ط��ű�ʾ����ͬ����
��2��T��X����Ԫ����ɵ�һ�ֻ�����M����Ҫ�Ļ���ԭ�ϣ��������IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����M�����ЦҼ��ͦм��ĸ�����Ϊ______��
��3����amolQ������bmolR������ɵĹ�������Ͷ�뵽������ˮ�У���������ȫ�ܽ����յõ�������Һ����ͬʱ��������������ʵ���Ϊ______mol����a��b��ʾ������a=bʱ��д����Ӧ�����ӷ���ʽ______��
��4��Y2T4������Yԭ�ӹ�����ӻ�����Ϊ______��Y2T4��������������������һ���⻯����⻯�����Է�������Ϊ43.0�����е�ԭ�ӵ���������Ϊ0.977��д��Y2T4�������ᷴӦ�Ļ�ѧ����ʽ______��
��5��YT3�ķе�Ȼ�����XT4�ĸߣ�����Ҫԭ���ǣ�______��
��6��P�ĵ�����T��Z�γɵļ��м��Ի�ѧ�����зǼ��Ի�ѧ�����ۻ����ﷴӦ�����ӷ���ʽΪ______��
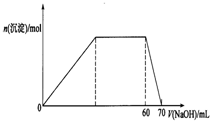
��7����������Ԫ���γɵ����е����ֿ���������γ�һ�ָ��Σ�����ε�Ũ��Һ����μ���0.1mol/L����������Һ����������������Һ�ļ��룬���������Ĺ�ϵ��ͼ����ø��εĻ�ѧʽΪ______��
| Ԫ�� | �� �� �� Ϣ |
| T | Tԭ�����������������������ֱ�����ԭ��������� |
| X | X�Ļ�̬ԭ���е���ռ������������ͬ��ԭ�ӹ������ÿ�ֹ���еĵ�������ͬ |
| Z | Z���γ�Z2��Z3������̬���� |
| Q | �ڸ�Ԫ�����������У�Q�Ļ�̬ԭ�ӵĵ�һ��������С |
| R | R������Ϊ�������ӣ������Ӱ뾶�����������ͬ���Ӳ�ṹ�����а뾶��С�� |
| P | Z��P��ͬ���ڵ�����Ԫ�أ�R��P��ԭ������֮��Ϊ30 |
| W | W��һ�ֺ��ص�������Ϊ56��������Ϊ30 |
��2��T��X����Ԫ����ɵ�һ�ֻ�����M����Ҫ�Ļ���ԭ�ϣ��������IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����M�����ЦҼ��ͦм��ĸ�����Ϊ______��
��3����amolQ������bmolR������ɵĹ�������Ͷ�뵽������ˮ�У���������ȫ�ܽ����յõ�������Һ����ͬʱ��������������ʵ���Ϊ______mol����a��b��ʾ������a=bʱ��д����Ӧ�����ӷ���ʽ______��
��4��Y2T4������Yԭ�ӹ�����ӻ�����Ϊ______��Y2T4��������������������һ���⻯����⻯�����Է�������Ϊ43.0�����е�ԭ�ӵ���������Ϊ0.977��д��Y2T4�������ᷴӦ�Ļ�ѧ����ʽ______��
��5��YT3�ķе�Ȼ�����XT4�ĸߣ�����Ҫԭ���ǣ�______��
��6��P�ĵ�����T��Z�γɵļ��м��Ի�ѧ�����зǼ��Ի�ѧ�����ۻ����ﷴӦ�����ӷ���ʽΪ______��

��7����������Ԫ���γɵ����е����ֿ���������γ�һ�ָ��Σ�����ε�Ũ��Һ����μ���0.1mol/L����������Һ����������������Һ�ļ��룬���������Ĺ�ϵ��ͼ����ø��εĻ�ѧʽΪ______��
T��X��Y��Z��Q��R��P��WΪ���ڱ�ǰ������Ԫ�أ�ԭ���������ε�����Tԭ�����������������������ֱ�����ԭ��������ȣ���T��ԭ������Ϊ1��TΪ��Ԫ�أ�X�Ļ�̬ԭ���е���ռ������������ͬ��ԭ�ӹ������ÿ�ֹ���еĵ�������ͬ�����������Ų�Ϊ1s22s22p2����XΪ̼Ԫ�أ�Z���γ�Z2��Z3������̬���ʣ���ZΪ��Ԫ�أ�Yԭ����������̼Ԫ������Ԫ��֮�䣬��YΪ��Ԫ�أ�R��P��ԭ������֮��Ϊ30����Ԫ����PԪ�ز�ͬ���ڵ�����Ԫ�أ���PΪ��A����A����P�����ܴ��ڵ������ڣ���P���ڵ������ڣ���R��ԭ��������С��R������Ϊ�������ӣ������Ӱ뾶�����������ͬ���Ӳ�ṹ�����а뾶��С�ģ���RΪAlԪ�أ���P��ԭ������Ϊ30-13=17����PΪClԪ�أ�Q��ԭ������������Ԫ��С��AlԪ�أ��ڸ�Ԫ�����������У�Q�Ļ�̬ԭ�ӵĵ�һ��������С����Q���ڵ������ڣ�ΪNaԪ�أ�W��һ�ֺ��ص�������Ϊ56��������Ϊ30��������Ϊ56-30=26����WΪFeԪ�أ�
��1��ͬ����������ҵ�һ������������ƣ���NԪ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�������ϵͣ�ʧȥ������Ҫ�������ϴ�һ�����ܸ�������Ԫ�أ��ʵ�һ������N��O��C��
�ʴ�Ϊ��N��O��C��
��2��H��C����Ԫ����ɵ�һ�ֻ�����M����Ҫ�Ļ���ԭ�ϣ��������IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����MΪC2H4�������к���4��C-H����1��C=C˫���������ǦҼ���˫������1���Ҽ���1���м�����C2H4�����ЦҼ��ͦм��ĸ�����Ϊ5��1��
�ʴ�Ϊ��5��1��
��3����amolNa������bmolAl������ɵĹ�������Ͷ�뵽������ˮ�У���������ȫ�ܽ����յõ�������Һ��
���ݵ���ת���غ��֪�����ɵ����������ʵ���Ϊ
=
mol����a=bʱ������ǡ�÷�Ӧ����NaAlO2����Ӧ�����ӷ���ΪNa+Al+2H2O=Na++AlO2-+2H2����
�ʴ�Ϊ��
��Na+Al+2H2O=Na++AlO2-+2H2����
��4��N2H4������Nԭ�ӳ�2��N-H����1��N-N��������1�Թ¶Ե��Ӷԣ�Nԭ���ӻ������ĿΪ4��Nԭ�Ӳ�ȡsp3�ӻ���N2H4��������������������һ���⻯����⻯�����Է�������Ϊ43.0�����е�ԭ�ӵ���������Ϊ0.977������⻯��Nԭ����ĿΪ
=3����ԭ����ĿΪ
=1�����⻯��ΪHN3��ͬʱ����ˮ���÷�Ӧ����ʽΪN2H4+HNO2=HN3+2H2O���ʴ�Ϊ��sp3�ӻ���N2H4+HNO2=HN3+2H2O��
��5��NH3���Ӽ���������NH3�ķе�Ȼ�����CH4�ĸߣ��ʴ�Ϊ��NH3���Ӽ���������
��6��T��Z�γɵļ��м��Ի�ѧ�����зǼ��Ի�ѧ�����ۻ�����ΪH2O2��������H2O2����HCl����������Ӧ�����ӷ���ʽΪCl2+H2O2=2H++2Cl-+O2����
�ʴ�Ϊ��Cl2+H2O2=2H++2Cl-+O2����
��7����ͼ��֪���ø������������Ʒ�Ӧ���ɳ������ó��������������ƣ���Ӧ����Al3+���ܽ������������ĵ��������Ƶ����ʵ���Ϊ10mol����n��Al3+��=n[Al��OH��3]=n��NaOH��=0.1L��0.1mol/L=0.01mol��������������ҪNaOH�����ʵ���Ϊ0.3mol�����������������ʱ�������μ��������ƣ���������û�������ܽ⣬������Ӧ����NH4+����n��NH4+��=0.7L��0.1mol/L-0.3mol-0.1mol=0.3mol��������n��NH4+����n��Al3+��=0.3mol��0.1mol=3��1���ʸø��εĻ�ѧʽΪ��NH4��3Al��SO4��3��
�ʴ�Ϊ����NH4��3Al��SO4��3��
��1��ͬ����������ҵ�һ������������ƣ���NԪ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�������ϵͣ�ʧȥ������Ҫ�������ϴ�һ�����ܸ�������Ԫ�أ��ʵ�һ������N��O��C��
�ʴ�Ϊ��N��O��C��
��2��H��C����Ԫ����ɵ�һ�ֻ�����M����Ҫ�Ļ���ԭ�ϣ��������IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����MΪC2H4�������к���4��C-H����1��C=C˫���������ǦҼ���˫������1���Ҽ���1���м�����C2H4�����ЦҼ��ͦм��ĸ�����Ϊ5��1��
�ʴ�Ϊ��5��1��
��3����amolNa������bmolAl������ɵĹ�������Ͷ�뵽������ˮ�У���������ȫ�ܽ����յõ�������Һ��
���ݵ���ת���غ��֪�����ɵ����������ʵ���Ϊ
| amol��1+bmol��3 |
| 2 |
| a+3b |
| 2 |
�ʴ�Ϊ��
| a+3b |
| 2 |
��4��N2H4������Nԭ�ӳ�2��N-H����1��N-N��������1�Թ¶Ե��Ӷԣ�Nԭ���ӻ������ĿΪ4��Nԭ�Ӳ�ȡsp3�ӻ���N2H4��������������������һ���⻯����⻯�����Է�������Ϊ43.0�����е�ԭ�ӵ���������Ϊ0.977������⻯��Nԭ����ĿΪ
| 43��0.977 |
| 14 |
| 43-14��2 |
| 1 |
��5��NH3���Ӽ���������NH3�ķе�Ȼ�����CH4�ĸߣ��ʴ�Ϊ��NH3���Ӽ���������
��6��T��Z�γɵļ��м��Ի�ѧ�����зǼ��Ի�ѧ�����ۻ�����ΪH2O2��������H2O2����HCl����������Ӧ�����ӷ���ʽΪCl2+H2O2=2H++2Cl-+O2����
�ʴ�Ϊ��Cl2+H2O2=2H++2Cl-+O2����
��7����ͼ��֪���ø������������Ʒ�Ӧ���ɳ������ó��������������ƣ���Ӧ����Al3+���ܽ������������ĵ��������Ƶ����ʵ���Ϊ10mol����n��Al3+��=n[Al��OH��3]=n��NaOH��=0.1L��0.1mol/L=0.01mol��������������ҪNaOH�����ʵ���Ϊ0.3mol�����������������ʱ�������μ��������ƣ���������û�������ܽ⣬������Ӧ����NH4+����n��NH4+��=0.7L��0.1mol/L-0.3mol-0.1mol=0.3mol��������n��NH4+����n��Al3+��=0.3mol��0.1mol=3��1���ʸø��εĻ�ѧʽΪ��NH4��3Al��SO4��3��
�ʴ�Ϊ����NH4��3Al��SO4��3��

��ϰ��ϵ�д�
�����Ŀ
