��Ŀ����
1�����ڼס��ҡ�����������������Һ��������Ϣ���ٷֱ�NH4+��Na+��Al3+��Ba2+��Ag+��NO3-��Cl-��SO42-��Br-��CO32-�����еĸ�һ����ɣ����ظ�����
�����мס�������������Һ�����ԣ�����Һ�ʼ��ԣ�
�ۼס��ҷ�Ӧ���ɰ�ɫ���������壬���ɷֱ���ס��ҡ�����Ӧ���ɰ�ɫ������
��ش��������⣺
��1���û�ѧʽ��ʾ�������ʣ���Na2CO3����BaCl2��
��2���������ӷ���ʽ��ʾ����Һ�����Ե�ԭ��NH4++H2O?NH3•H2O+H+��
��3���������ӷ���ʽ��ʾ�����ҵķ�Ӧ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����
��4���������Һ�м������ӵķ���������Ҫ�������ᣬȻ���ټ����Ȼ�����Һ��������ְ�ɫ������˵������SO42-��
���� ����Ag+ֻ�ܺ�NO3-���棬����һ���������������ݢۿ�֪�������ð�ɫ������Al��OH��3��������CO2�������ٸ��ݢڿ�֪������Na2CO3�����к���Al3+������Ϊ���ɷֱ���ס��ҡ�����Ӧ���ɰ�ɫ���������к���Ba2+���ס�������������Һ�����ԣ��������ԣ����Զ���BaCl2�������Al2��SO4��3������AgNO3���������NH4Br��
��� �⣺����Ag+ֻ�ܺ�NO3-���棬����һ���������������ݢۿ�֪�������ð�ɫ������Al��OH��3��������CO2�������ٸ��ݢڿ�֪������Na2CO3�����к���Al3+������Ϊ���ɷֱ���ס��ҡ�����Ӧ���ɰ�ɫ���������к���Ba2+���ס�������������Һ�����ԣ��������ԣ����Զ���BaCl2�������Al2��SO4��3������AgNO3���������NH4Br��
��1��������������֪����ΪNa2CO3����Ϊ��BaCl2���ʴ�Ϊ��Na2CO3��BaCl2��
��2��NH4Br��ǿ�������Σ�NH4+ˮ�������ԣ����ӷ���ʽΪ��NH4++H2O?NH3•H2O+H+��
�ʴ�Ϊ��NH4++H2O?NH3•H2O+H+��
��3��������ˮ�������ԣ�CO32-ˮ���Լ��ԣ�������ٽ������Է�Ӧ���ӷ���ʽΪ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����
�ʴ�Ϊ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����
��4������SO42-ʱ������Ҫ�������ᣬ���ų�CO32-�ȸ��ţ�Ȼ���ټ����Ȼ�����Һ��������ְ�ɫ������˵������SO42-��
�ʴ�Ϊ������Ҫ�������ᣬȻ���ټ����Ȼ�����Һ��������ְ�ɫ������˵������SO42-��
���� ���⿼��������ƶϣ��漰����ˮ�⡢���ӹ��桢���ӷ�Ӧ�����Ӽ�������ݣ�������ѧ���ķ��������������飬��Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��ϩ�ı���ģ�ͣ�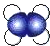 | B�� | 3��3-��������ļ���ʽ��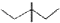 | ||
| C�� | �Լ����״��Ľṹ��ʽ��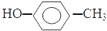 | D�� | ȩ���Ľṹ��ʽ��-CHO |
| A�� | ������믣�XeF6�� | B�� | �����ᣨHClO�� | C�� | ��������BF3�� | D�� | ���� ��N2�� |
 �����й��ڸ����ʵ�˵����ȷ���ǣ�������
�����й��ڸ����ʵ�˵����ȷ���ǣ�������| A�� | �����ʽΪC15H21O4 | |
| B�� | ������ֻ�������ּ��Թ��ۼ� | |
| C�� | ���ܷ����Ӿ۷�Ӧ�����ܷ���������Ӧ | |
| D�� | ������FeCl3��Һ������ɫ��Ӧ������ʹ����KMnO4��Һ��ɫ |
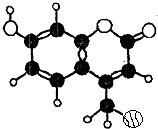 ij�ּ���Ⱦ�ϣ�Ӧ���ڿɵ�гȾ�ϼ�����������C��H��O����Ԫ����ɣ���һ�����ķ����������ͼ��ʾ�������йظ�������������ȷ���ǣ�������
ij�ּ���Ⱦ�ϣ�Ӧ���ڿɵ�гȾ�ϼ�����������C��H��O����Ԫ����ɣ���һ�����ķ����������ͼ��ʾ�������йظ�������������ȷ���ǣ��������ٸ÷��ӵĺ˴Ź���������5�����շ�
��������ˮ����ȡ����Ӧ
��1mol�������������4molH2�ӳ�
����ʹ����KMnO4��Һ��ɫ
��1mol������������뺬3mol NaOH����Һ��Ӧ��
| A�� | �٢ڢ� | B�� | �ۢܢ� | C�� | �ڢۢ� | D�� | �ڢۢܢ� |
| A�� |  2-�һ����� | B�� |  3-����-3-��ϩ | C�� |  2��4-���������� | D�� |  2��3-������ |
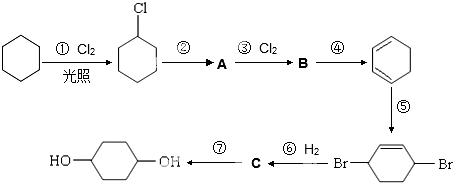
 ��
��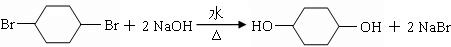 ��
��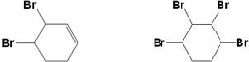 ��
�� +O2$��_{��}^{����}$
+O2$��_{��}^{����}$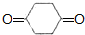 +2H2O��
+2H2O��
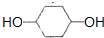
 ��B�Ľṹ��ʽ
��B�Ľṹ��ʽ ��
�� ��
�� ����Ӧ���ͣ�ȡ����Ӧ��
����Ӧ���ͣ�ȡ����Ӧ��