��Ŀ����
��8�֣�����ʹ�õ�ȼ�ϣ��ִ����ú����Һ��ʯ������ú������Ҫ�ɷ���CO��H2�Ļ�����壬����ú̿��ˮ������Ӧ�Ƶã����ֳ�ˮú����
��1����д����ȡˮú������Ҫ��ѧ����ʽ____________________________.
��2��Һ��ʯ��������Ҫ�ɷ��DZ��飬����ȼ�յ��Ȼ�ѧ����ʽΪ
C3H8(g) +5O2(g)== 3CO2(g) + 4H2O(l)����H=�C2220.0 kJ��![]() ,
,
��֪CO����ȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g) +1/2O2(g) == CO2(g) ����H=�C282.57kJ��![]() ,
,
�ԱȽ�ͬ������C3H8��COȼ�գ�������������ֵԼΪ_________��1��
��3����֪����ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g)+ O2(g) == 2H2O(l)����H=�C571.6 kJ��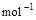 ,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
��4��������δ������Դ������Դ�ḻ֮�⣬�����е��ŵ���____________________
��1��C+H2O(g)== CO+H2 ��2��5.0 ��3��2.8
��4��������ʱ���ȶࣻ��������Ⱦ
����:

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���8�֣�����ʹ�õ�ȼ�ϣ��ִ����ú����Һ��ʯ������ú������Ҫ�ɷ���CO��H2�Ļ�����壬����ú̿��ˮ������Ӧ�Ƶã����ֳ�ˮú����
��1����д����ȡˮú������Ҫ��ѧ����ʽ____________________________.
��2��Һ��ʯ��������Ҫ�ɷ��DZ��飬����ȼ�յ��Ȼ�ѧ����ʽΪ
C3H8(g) +5O2(g)
== 3CO2(g) + 4H2O(l)����H=�C2220.0 kJ��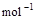 ,
,
��֪CO����ȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g) +1/2O2(g) == CO2(g) ����H=�C282.57kJ��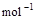 ,
,
�ԱȽ�ͬ������C3H8��COȼ�գ�������������ֵԼΪ_________��1��
��3����֪����ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g)
+ O2(g) == 2H2O(l)����H=�C571.6 kJ��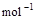 ,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
��4��������δ������Դ������Դ�ḻ֮�⣬�����е��ŵ���____________________
 ,
,