��Ŀ����
�����������BHDBT���е��۵㡢���������ص㣬��������TNT��Ϊ��������ըҩ����ϳ�·�����£�
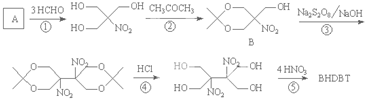
��֪����1��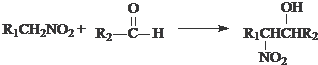 ����R1CH2NO2��������Ϊȩ����Ҳ�ܷ������Ʒ�Ӧ��
����R1CH2NO2��������Ϊȩ����Ҳ�ܷ������Ʒ�Ӧ��
��2��1mol R-NH2�������������Ʒ�Ӧ�ų�1mol H2��
�ش��������⣺
��1������A�Ľṹ��ʽΪ
��2����Ʒ�Ӧ�ڵ�Ŀ����
��3��д����Ӧ�ݵĻ�ѧ����ʽ
 ��
��
��4������ȩ���״�Ϊԭ�ϣ��ϳɾ�2-��ϩ������� �����ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�CH3CH2OH
�����ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�CH3CH2OH
H2C=CH2
BrH2C-CH2Br��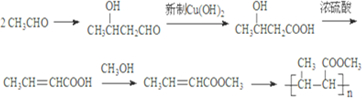
 ��
��

��֪����1��
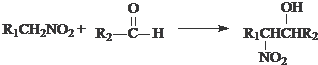 ����R1CH2NO2��������Ϊȩ����Ҳ�ܷ������Ʒ�Ӧ��
����R1CH2NO2��������Ϊȩ����Ҳ�ܷ������Ʒ�Ӧ����2��1mol R-NH2�������������Ʒ�Ӧ�ų�1mol H2��
�ش��������⣺
��1������A�Ľṹ��ʽΪ
CH3NO2
CH3NO2
����2����Ʒ�Ӧ�ڵ�Ŀ����
���������е������ǻ�
���������е������ǻ�
���÷�Ӧ����һ����ΪH2O
H2O
����3��д����Ӧ�ݵĻ�ѧ����ʽ


��4������ȩ���״�Ϊԭ�ϣ��ϳɾ�2-��ϩ�������
 �����ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�CH3CH2OH
�����ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�CH3CH2OH| Ũ���� |
| 170�� |
| Br |


��������Ӧ��Ϊ�ӳɷ�Ӧ���������Ϣ�Լ������֪AΪCH3NO2������Ӧ�ڢۢܺ��ֿ�����2��-OH��˵����Ӧ�ڿ������ǻ������ã����ݷ�Ӧ����������֪��Ӧ�ڻ�Ӧ����ˮ����Ӧ��Ϊ������Ӧ������BHDBT��ˮ������ȩ���״�Ϊԭ�ϣ��ϳɾ�2-��ϩ������� ����Ӧ������CH3CH=CHCOOCH3���������Ʒ��ƶϺϳ�·�ߣ�
����Ӧ������CH3CH=CHCOOCH3���������Ʒ��ƶϺϳ�·�ߣ�
 ����Ӧ������CH3CH=CHCOOCH3���������Ʒ��ƶϺϳ�·�ߣ�
����Ӧ������CH3CH=CHCOOCH3���������Ʒ��ƶϺϳ�·�ߣ�����⣺��1����Ӧ��Ϊ�ӳɷ�Ӧ���������Ϣ�Լ������֪AΪCH3NO2���ʴ�Ϊ��CH3NO2��
��2������Ӧ�ڢۢܺ��ֿ�����2��-OH��˵����Ӧ�ڿ������ǻ������ã����ݷ�Ӧ����������֪��Ӧ�ڻ�Ӧ����ˮ���ʴ�Ϊ�����������е������ǻ���H2O��
��3����Ӧ��Ϊ������Ӧ������BHDBT��ˮ������ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��4������ȩ���״�Ϊԭ�ϣ��ϳɾ�2-��ϩ������� ����Ӧ������CH3CH=CHCOOCH3������CH3CH=CHCOOH��CH3OH���ɣ�
����Ӧ������CH3CH=CHCOOCH3������CH3CH=CHCOOH��CH3OH���ɣ�
CH3CH=CHCOOH����CH3CH��OH��CH2COOH��������ȩ���Ϸ�Ӧ���ɣ���Ӧ����Ϊ ��
��
�ʴ�Ϊ�� ��
��
��2������Ӧ�ڢۢܺ��ֿ�����2��-OH��˵����Ӧ�ڿ������ǻ������ã����ݷ�Ӧ����������֪��Ӧ�ڻ�Ӧ����ˮ���ʴ�Ϊ�����������е������ǻ���H2O��
��3����Ӧ��Ϊ������Ӧ������BHDBT��ˮ������ʽΪ
 ��
���ʴ�Ϊ��
 ��
����4������ȩ���״�Ϊԭ�ϣ��ϳɾ�2-��ϩ�������
 ����Ӧ������CH3CH=CHCOOCH3������CH3CH=CHCOOH��CH3OH���ɣ�
����Ӧ������CH3CH=CHCOOCH3������CH3CH=CHCOOH��CH3OH���ɣ�CH3CH=CHCOOH����CH3CH��OH��CH2COOH��������ȩ���Ϸ�Ӧ���ɣ���Ӧ����Ϊ
 ��
���ʴ�Ϊ��
 ��
�����������⿼���л���ĺϳ����ƶϣ��ۺϿ���ѧ�������������ƶ��������ۺ����û�ѧ֪ʶ��������Ϊ�߿��������ͣ�ע�Ȿ��������Ʒ��ƶϣ����������Ϣ����Ŀ�ѶȲ����״���Ϊ��4����ע���������Ʒ����

��ϰ��ϵ�д�
�����Ŀ