��Ŀ����
����Ŀ����֪A��B��C��D��E��F��G��H��I�������ʣ�����A��B��D��E��ɫ��Ӧ��Ϊ��ɫ(����ɫ�ܲ���)��G��F�ǵ��ʣ������Ϊ�����H��һ�ֵ���ɫ���壬���ǵ�ת����ϵ��ͼ��ʾ����ش�
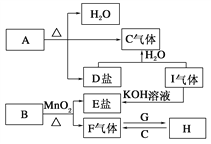
��1������G���ӽṹʾ��ͼ________��
��2��д��B��I�Ļ�ѧʽB________��I________��
��3��д��H��һ����;__________________________________��
��4��д��A���ȷֽ�Ļ�ѧ����ʽ_________________________��
��5����10g��C6H12O6�������г��ȼ�գ�������ȫ����������H��ַ�Ӧ����Ӧ���������____________g��
���𰸡�![]() KClO3 HCl �����ں�������к�DZˮͧ����Ϊ��������Դ(�������Ư��֯���) 2KHCO3
KClO3 HCl �����ں�������к�DZˮͧ����Ϊ��������Դ(�������Ư��֯���) 2KHCO3![]() K2CO3��H2O��CO2�� 10
K2CO3��H2O��CO2�� 10
��������
A��B��C��D��E��F��G��H��I�������ʣ�����A��B��D��E��ɫ��Ӧ��Ϊ��ɫ������ɫ�ܲ�������˵�����м�Ԫ�أ�F�ǵ��ʣ�B�Ͷ��������ڼ����������������嵥�ʣ���BΪKClO3��FΪO2��EΪKCl��G�ǵ��ʣ�HΪ����ɫ���壬Na��������ȼ�����ɹ������ƣ�����G��Na��HΪNa2O2��C�����壬�������ƺͶ�����̼��Ӧ��������������C��CO2��̼���κ��ᷴӦ���ɶ�����̼��A���ȷֽ����ɶ�����̼��ˮ��̼���Σ�A�к��м�Ԫ�أ�����A��KHCO3��D��K2CO3��I��KOH��Ӧ����KCl������IΪHCl���壬
��1��Gԭ�Ӻ�����3�����Ӳ㡢������������1����ԭ��ʧȥ1���������������ӣ����������ӽṹʾ��ͼΪ��![]() ����2��ͨ�����Ϸ���֪��B��I�Ļ�ѧʽ�ֱ�ΪKClO3��HCl����3��H�ǹ������ƣ�H��һ����;�ǿ����ں�����ߺ�DZˮͧ����Ϊ��������Դ������Ư��֯���4�����������£�̼����طֽ�����̼��ء�������̼��ˮ����Ӧ����ʽΪ2KHCO3
����2��ͨ�����Ϸ���֪��B��I�Ļ�ѧʽ�ֱ�ΪKClO3��HCl����3��H�ǹ������ƣ�H��һ����;�ǿ����ں�����ߺ�DZˮͧ����Ϊ��������Դ������Ư��֯���4�����������£�̼����طֽ�����̼��ء�������̼��ˮ����Ӧ����ʽΪ2KHCO3![]() K2CO3��H2O��CO2������5���������ƺͶ�����̼��Ӧ�ķ���ʽΪ2Na2O2+2CO2=2Na2CO3+O2����������ת��Ϊ̼����ʱ�������������ӵ����൱��CO������������ˮ��Ӧ�ķ���ʽΪ��2Na2O2+2H2O=4NaOH+O2������������ת��Ϊ��������ʱ�������������ӵ����൱��H2��C6H12O6�ɿ���ʽ��Ϊ(CO)6(H2)6�� 10g��C6H12O6�������г��ȼ�գ�������ȫ����������Na2O2��ַ�Ӧ�����ӵ������൱��C6H12O6����������Ϊ10g��
K2CO3��H2O��CO2������5���������ƺͶ�����̼��Ӧ�ķ���ʽΪ2Na2O2+2CO2=2Na2CO3+O2����������ת��Ϊ̼����ʱ�������������ӵ����൱��CO������������ˮ��Ӧ�ķ���ʽΪ��2Na2O2+2H2O=4NaOH+O2������������ת��Ϊ��������ʱ�������������ӵ����൱��H2��C6H12O6�ɿ���ʽ��Ϊ(CO)6(H2)6�� 10g��C6H12O6�������г��ȼ�գ�������ȫ����������Na2O2��ַ�Ӧ�����ӵ������൱��C6H12O6����������Ϊ10g��

����Ŀ���±������������г��������ʣ������г������ǵ�(��Ҫ���ɷ֡�
��� | �� | �� | �� | �� | �� | �� | �� |
���� | �ƾ� | ���� | ��� | ʳ�� | ͭ���� | �������� | �մ� |
��Ҫ�ɷ� | CH3CH2OH | CH3COOH | NaOH | NaCl | Cu | SO2 | Na2CO3 |
��1������Ա��Т����ߵ���Ҫ�ɷֽ��з���(���ţ������ڵ���ʵ���______�����ڷǵ����_______��
��2�������ڵ�ˮ��Һ��߷�Ӧ�����ӷ���ʽ______________________��
��3��ijͬѧ�âݺ�Ũ���Ṳ�����Ʊ��ޣ���ѧ����ʽΪ��Cu+2H2SO4(Ũ��![]() CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
�����õ����ű������ת�Ƶ����____________��
��ŨH2SO4���ֳ����������ǣ�_______��������ת��0.1molʱ�����������������ʵ���Ϊ_______��
��4����ͼ��ʾijͬѧ����480mL 0.5mol/L ��NaOH��Һ�IJ��ֲ���ʾ��ͼ�������д������_______���������������Ƶ���Һ��Ҫ���Ũ��Ҫ_________ (����ƫ��������ƫ����������Ӱ������������Ӧ��ȡNaOH________g��

����Ŀ������ͼװ����ȡ���ᴿ���ռ����е���������(a��b��c��ʾ��Ӧ�����м�����Լ�)�����п��е���(����)

���� | a | b | c | |
A | NO2 | Ũ���� | ͭƬ | NaOH��Һ |
B | SO2 | Ũ���� | Cu | ����KMnO4��Һ |
C | NH3 | Ũ��ˮ | ��ʯ�� | ��ʯ�� |
D | O2 | ˫��ˮ | MnO2 | Ũ���� |
A. A B. B C. C D. D