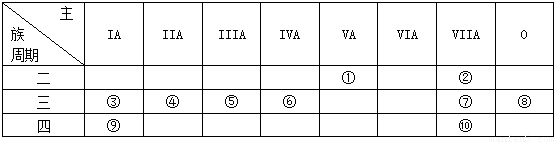
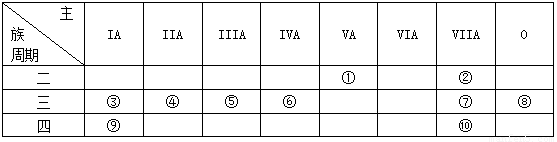
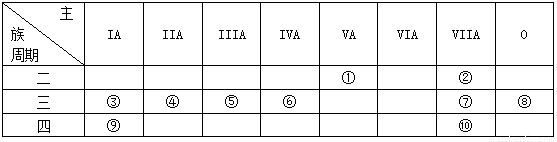
��Ŀ����
��1��ʵ�鳣ʶ��գ���ȡ����������Ҫ�IJ���������
��2���������������ʣ�
��1.1molˮ���ں�3.01��1023�����ӵ�CO2��������10mL 0.1mol/L Na2CO3��Һ�õ��Ĺ����ĩ����6g H2��
ѡ�����ʶ�Ӧ�ķ�����գ�
��������������
��3��������CO��CO2�����ʵ���֮��Ϊ
��2�������������������ʵ����������壻����ÿ����������ԭ�ӵĸ����������ԭ�ӵ����ʵ������ɷֱ����ÿ�����ʵ�����������n=
| m |
| M |
| N |
| N A |
| V |
| Vm |
��3������n=
| m |
| M |
| N |
| N A |
| V |
| Vm |
�ʴ�Ϊ����Һ©�������У����Ƭ������ǯ��
��2������£�ֻ�ж�����̼�����������壬��3.01��1023�����ӵ�CO2�����ʵ�����0.5mol����6g H2�����ʵ�����3mol���������������Ǣܣ�
��1.1molˮ������ԭ�ӵ����ʵ�����1.1mol��
�ں�3.01��1023�����ӵ�CO2������ԭ�ӵ����ʵ�����1mol��
������10mL 0.1mol/L Na2CO3��Һ�õ��Ĺ����ĩ������ԭ�ӵ����ʵ�����0.003mol��
��6g H2��������ԭ�ӣ�
������Oԭ�Ӹ��������Ǣ٣�
��1.1molˮ��������1.1��18=19.8g��
�ں�3.01��1023�����ӵ�CO2��������0.5��44=22g��
������10mL 0.1mol/L Na2CO3��Һ�õ��Ĺ����ĩ��������0.003mol��106=0.318g��
��6g H2��������6g��
���������Ǣڣ�
�ʴ�Ϊ���ܣ��٣��ڣ�
��3����CO��CO2��������Ϊmg����n��CO��=
| m |
| 28 |
| m |
| 44 |
�����ʵ���֮��Ϊ��
| m |
| 28 |
| m |
| 44 |
��������ԭ�Ӹ�����Ϊ��11��7��2=11��14��
��ͬ�����µ������Ϊ�������ʵ���֮�ȣ�Ϊ11��7��
�ʴ�Ϊ��11��7��11��7��11��14��

ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���

�밴Ҫ����գ�
��1������Bװ�ÿ���ȡ��������____________��д�����ּ��ɣ���
��2��A��C��E�������װ�ÿ�������ȡCl2��������ص�����ʵ�飮
�����ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ
�飬ʵ����������������£�
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | ��Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м���̼�����Ʒ�ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ�IJ���������� |
��������ʵ���Ľ����Ƿ������������������˵������________________
________________________________.
������������װ�����һ����ʵ����֤Cl����Br���Ļ�ԭ��ǿ�����ֱ�ָ���ס��ҡ�����ʢ�ŵ��Լ���ʵ�������ۣ�____________
��3��B��D��Eװ����������B��ʢװŨ�����ͭƬ�������п����ϰ��ϣ������Ƶò�����NO2�й�ʵ�飮
��B�з�����Ӧ�Ļ�ѧ����ʽΪ______________________________________
������Dװ����֤NO2��ˮ�ķ�Ӧ�����������Ϊ���ȹر�ֹˮ��________���ٴ�ֹˮ��________��ʹ�ձ��е�ˮ�����Թܶ��IJ�����________________��
���Թܶ��е�NO2��ˮ��ַ�Ӧ�������Թ��ڻ���ͨ��һ������������ֱ���Թ�ȫ������ˮ����������Һ�����ʵ����ʵ���Ũ����________�����尴��״�����㣩��
����С�12�� ��ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���
��ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���

�밴Ҫ����գ�
��1������Bװ�ÿ���ȡ��������______________________��д�����ּ��ɣ���
��2��A��C��E�������װ�ÿ�������ȡCl2��������ص�����ʵ�飮
���ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ
�飬ʵ����������������£�
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | ��Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м���̼�����Ʒ�ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ�IJ���������� |