��Ŀ����
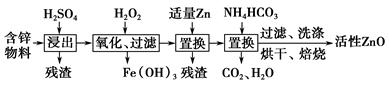
��ҵ���ú�п����(��FeO��CuO������)���Ƶû���ZnO���������£�
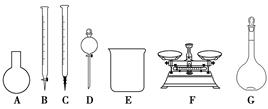
(1)���������У������õ���60%H2SO4(1.5 g��cm��3)����������H2SO4 100 mL��Ҫ18.4 mol��L��1��ŨH2SO4________ mL(����һλС��)��
(2)����������H2O2����Fe(OH)3�������֣�û��Cu(OH)2�������֣�����Һ��c(Fe3��)��2.6��10��18 mol��L��1������Һ��c(Cu2��)��ȡֵ��Χ��________mol��L��1��(��֪Ksp[Fe(OH)3]��2.6��10��39��
Ksp[Cu(OH)2]��2.2��10��20)
(3)����NH4HCO3�����ɵij�������̬��ΪZna(OH)b(CO3)c(a��b��cΪ������)�����ּ�ʽ̼��пA��B�Ļ���A��a��5��b��6�������ɼ�ʽ̼��пA�Ļ�ѧ����ʽΪ__________________________________________________��
(4)ȡϴ�ӡ���ɺ�ļ�ʽ̼��пA��B�Ļ����49.70 g�������ʵ���Ϊ0.10 mol�����±�����ȫ�ֽ�õ�37.26 g ZnO��3.584 L CO2(��״����)��ˮ��ͨ�����������ʽ̼��пB�Ļ�ѧʽ��

(1)���������У������õ���60%H2SO4(1.5 g��cm��3)����������H2SO4 100 mL��Ҫ18.4 mol��L��1��ŨH2SO4________ mL(����һλС��)��
(2)����������H2O2����Fe(OH)3�������֣�û��Cu(OH)2�������֣�����Һ��c(Fe3��)��2.6��10��18 mol��L��1������Һ��c(Cu2��)��ȡֵ��Χ��________mol��L��1��(��֪Ksp[Fe(OH)3]��2.6��10��39��
Ksp[Cu(OH)2]��2.2��10��20)
(3)����NH4HCO3�����ɵij�������̬��ΪZna(OH)b(CO3)c(a��b��cΪ������)�����ּ�ʽ̼��пA��B�Ļ���A��a��5��b��6�������ɼ�ʽ̼��пA�Ļ�ѧ����ʽΪ__________________________________________________��
(4)ȡϴ�ӡ���ɺ�ļ�ʽ̼��пA��B�Ļ����49.70 g�������ʵ���Ϊ0.10 mol�����±�����ȫ�ֽ�õ�37.26 g ZnO��3.584 L CO2(��״����)��ˮ��ͨ�����������ʽ̼��пB�Ļ�ѧʽ��
(1)49.9(50.0Ҳ����) (2)��2.2��10��6
(3)5ZnSO4��10NH4HCO3=Zn5(OH)6(CO3)2����5(NH4)2SO4��8CO2����2H2O
(4)������0.1 mol�������ȫ�ֽ�õ�ZnO��CO2��H2O�����ʵ����ֱ�Ϊ0.46 mol��0.16 mol��0.3 mol
��֪1 mol�������ƽ����4.6 mol Zn��1.6 mol C��6 mol H����֪1 mol A�к�HΪ6 mol����CΪ2 mol����1 mol B�к�HΪ6 mol����CΪ1 mol������B�Ļ�ѧʽ���Ա�ʾΪZnx(OH)6CO3���ɻ��ϼ۴�����Ϊ��ó�x��4����B�Ļ�ѧʽΪZn4(OH)6CO3(���������ⷨ����)
(3)5ZnSO4��10NH4HCO3=Zn5(OH)6(CO3)2����5(NH4)2SO4��8CO2����2H2O
(4)������0.1 mol�������ȫ�ֽ�õ�ZnO��CO2��H2O�����ʵ����ֱ�Ϊ0.46 mol��0.16 mol��0.3 mol
��֪1 mol�������ƽ����4.6 mol Zn��1.6 mol C��6 mol H����֪1 mol A�к�HΪ6 mol����CΪ2 mol����1 mol B�к�HΪ6 mol����CΪ1 mol������B�Ļ�ѧʽ���Ա�ʾΪZnx(OH)6CO3���ɻ��ϼ۴�����Ϊ��ó�x��4����B�Ļ�ѧʽΪZn4(OH)6CO3(���������ⷨ����)
(1)V��100 mL��1.5 g��mL��1��60%��98 g��mol��1��18.4 mol��L��1��1 000 mL��L��1��49.9 mL��
������Һ����Fe(OH)3�������֣���c3(OH��)��c(Fe3��)��Ksp[Fe(OH)3]����Ksp[Fe(OH)3]��2.6��10��39��c(Fe3��)��2.6��10��18 mol��L��1������ʽ�ã�c(OH��)��1��10��7 mol��L��1���ٸ�����Һ��û��Cu(OH)2�������֣���c2(OH��)��
c(Cu2��)��Ksp[Cu(OH)2]����Ksp[Cu(OH)2]��2.2��10��20��c(OH��)��1��10��7
mol��L��1������ʽ�ã�c(Cu2��)��2.2��10��6 mol��L��1��
(3)����A��a��5��b��6,2a��b��2c(����غ�)�����c��2���ɴ�ȷ��A�Ļ�ѧʽΪZn5(OH)6(CO3)2���ٴ�����ͼ��֪������NH4HCO3�������ķ�Ӧ�з�Ӧ�ﻹ��ZnSO4����������Zn5(OH)6(CO3)2��CO2��H2O������(NH4)2SO4�������ݹ۲취����Ӧ��ѧ����ʽ��ƽ��
������Һ����Fe(OH)3�������֣���c3(OH��)��c(Fe3��)��Ksp[Fe(OH)3]����Ksp[Fe(OH)3]��2.6��10��39��c(Fe3��)��2.6��10��18 mol��L��1������ʽ�ã�c(OH��)��1��10��7 mol��L��1���ٸ�����Һ��û��Cu(OH)2�������֣���c2(OH��)��
c(Cu2��)��Ksp[Cu(OH)2]����Ksp[Cu(OH)2]��2.2��10��20��c(OH��)��1��10��7
mol��L��1������ʽ�ã�c(Cu2��)��2.2��10��6 mol��L��1��
(3)����A��a��5��b��6,2a��b��2c(����غ�)�����c��2���ɴ�ȷ��A�Ļ�ѧʽΪZn5(OH)6(CO3)2���ٴ�����ͼ��֪������NH4HCO3�������ķ�Ӧ�з�Ӧ�ﻹ��ZnSO4����������Zn5(OH)6(CO3)2��CO2��H2O������(NH4)2SO4�������ݹ۲취����Ӧ��ѧ����ʽ��ƽ��

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ