��Ŀ����
14������ʵ�����ݣ�д�����з�Ӧ���Ȼ�ѧ����ʽ����1��1molC2H4��g��������O2��g����Ӧ������CO2��g����H2O��l�����ų�1411kJ������
C2H4��g��+3O2��g���T2CO2��g��+2H2O��l������H=-1411kJ•mol-1��
��2��1molC2H5OH��l��������O2��g����Ӧ������CO2��g����H2O��l�����ų�1366.8kJ������
C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��l������H=-1366.8kJ•mol-1��
��3��2molAl��s��������O2��g��������Ӧ������Al2O3��s�����ų�1669.8kJ������
4Al��s��+3O2��g���T2Al2O3��s����H=-3339.6kJ•mol-1��
��4��18g������������O2��g����Ӧ������CO2��g����H2O��l�����ų�280.4kJ������
C6H12O6��s��+6O2��g���T6H2O��l��+6CO2��g������H=-2804kJ•mol-1��
��5��2.3gijҺ̬�л����һ������������ϵ�ȼ��ǡ����ȫȼ�գ�����2.7gˮ��2.24L CO2����״�������ų�68.35kJ������C2H6O��l��+3O2��g��-��2CO2��g��+3H2O��l������H=-1367kJ•mol-1��
���� �Ȼ�ѧ����ʽ����д����Ҫע����У����ʵ�״̬����Ӧ�ȵ���ֵ�뵥λ����Ӧ�ȵ���ֵ�뻯ѧ����ʽǰ���ϵ�������ȣ������Ȼ�ѧ����ʽ����д�������ش�
��� �⣺��1��1mol C2H4��g��������O2��g����Ӧ����CO2��g����H2O��l�����ų�1411kJ��������C2H4��g��+3O2��g���T2CO2 ��g��+2H2O��l����H=-1411 kJ•mol-1
���ʴ�Ϊ��C2H4��g��+3O2��g���T2CO2 ��g��+2H2O��l����H=-1411 kJ•mol-1��
��2��1mol C2H5OH��l��������O2��g����Ӧ����CO2��g����H2O��l�����ų�1366.8kJ��������C2H5OH��l��+3O2��g���T2CO2 ��g��+3H2O��l����H=-1366.8 kJ•mol-1���ʴ�Ϊ��C2H5OH��l��+3O2��g���T2CO2 ��g��+3H2O��l����H=-1366.8 kJ•mol-1��
��3��2molAl��s��������O2��g����Ӧ����Al2O3��s�����ų�1669.8kJ��������4Al��s��+3O2��g���T2Al2O3 ��s����H=-1669.8 kJ•mol-1��2=-3339.6kJ•mol-1���ʴ�Ϊ��4Al��s��+3O2��g���T2Al2O3 ��s����H=-3339.6kJ•mol-1��
��4��18g������������O2��g����Ӧ������180g�����Ƿ�Ӧ����CO2��g����H2O��l�����ų�������Ϊ2804kJ����Ӧ���Ȼ�ѧ����ʽ��C6H12O6��s��+6O2��g��=6CO2��g��+6H2O��l����H=-2804 kJ•mol-1���ʴ�Ϊ��C6H12O6��s��+6O2��g���T6H2O��l��+6CO2��g������H=-2804kJ•mol-1��
��5��2.3gijҺ̬�л����һ������������ϵ�ȼ��ǡ����ȫȼ�գ�����2.7gˮ���ʵ���=$\frac{2.7g}{18g/mol}$=0.15mol��2.24L CO2����״�������ʵ���=$\frac{2.24L}{22.4L/mol}$=0.1mol��n��C��=0.1mol��n��H��=0.3mol��n��O��=$\frac{2.3g-0.1mol��12g/mol-0.3mol��1g/mol}{16g/mol}$=0.05mol��n��C����n��H����n��O��=0.1mol��0.3mol��0.05mol=2��6��1����ѧʽΪC2H6O�����ʵ���Ϊ0.05mol�����ų�68.35kJ������1mol�л���ȼ�շ���1367KJ���Ȼ�ѧ����ʽΪ��C2H6O��l��+3O2��g���T2CO2��g��+3H2O��l����H=-1367kJ/mol��
�ʴ�Ϊ��C2H6O��l��+3O2��g��-��2CO2��g��+3H2O��l������H=-1367kJ•mol-1��
���� ������Ҫ�������Ȼ�ѧ����ʽ����д����Ҫע����У����ʵ�״̬����Ӧ�ȵ���ֵ�뵥λ����Ӧ�ȵ���ֵ�뻯ѧ����ʽǰ���ϵ�������ȣ�ͬʱ�������˷�Ӧ�ȵļ��㣬��Ŀ�ѶȲ���

| A�� | ����ʱ��Һ�泬����ֽ�ı�Ե | |
| B�� | �����Թ�������ʱ���Թܵײ���ƾ��Ƶ�о�Ӵ� | |
| C�� | ����ʱ��©���¶˽����ձ��ڱ� | |
| D�� | ���Թ��еμ�Һ��ʱ����ͷ�ιܽ����Թ��ڱ� |
| A�� | ��ʹ��ʪ�ĵ���KI��ֽ�����ɫ������һ����Cl2 | |
| B�� | ʵ����������ʱ���ñ���NaHCO3��Һ��Ũ���Ά������ | |
| C�� | ���ʹ�������������Ʒ�Ļ���ԭ�� | |
| D�� | ˮ���������������ϼ��ͷ���� |
| A�� | ���Ż�����ĭ�������� | |
| B�� | ����մ�����ϣ�Ӧ������NaOH��Һϴ | |
| C�� | Ƥ����մ��Ũ���ᣬ��������ˮ��ϴ | |
| D�� | �ƾ����������ᵹʧ���������ʪ������ |
| A�� | ��״���£�11.2 L�Ҵ��к��з��ӵ���ĿΪ0.5NA | |
| B�� | ��⾫��ͭ�Ĺ�����ת����NA�����ӣ�����������32 gͭ | |
| C�� | 2.8 g�ɵ�����һ����̼��ɵĻ�������к���ԭ�ӵ���ĿΪ0.1NA | |
| D�� | ���������£�20mL 10 mol/L Ũ����������ͭ��Ӧת�Ƶ�����Ϊ0.1NA |
 ����
����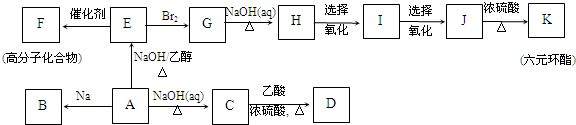
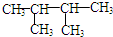 ��KΪ
��KΪ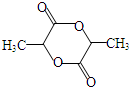 ��
��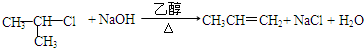 ��G��H
��G��H ��
��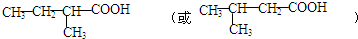 ��
��