��Ŀ����
��.����ȥ����Ĥ��þƬͶ���ˮ�У���Ӧ�Ļ�ѧ����ʽΪ?������������������������������������������?
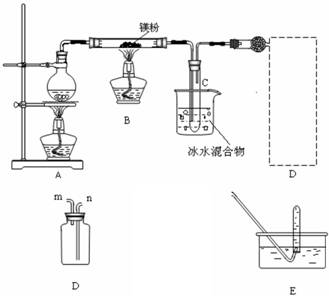
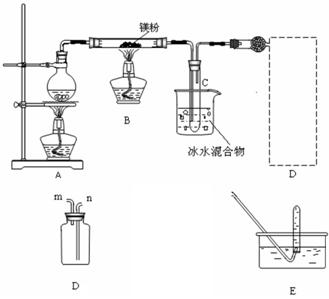
��.�������ȵ�þ����ˮ������Ӧ����������H2��ͬʱ�����ܵõ�����þ��ĩ��Ϊʵ����һ��Ӧ���ռ�һƿH2��ij����С���������ͼ��ʾ��װ�á�?
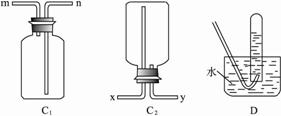
(1)����װ��C1���������������������ܿ�ӦΪ (�m����n��)������װ��C2���������������������ܿ�ӦΪ (�x����y��)��?
(2)ʵ�鿪ʼʱ��Ӧ�ȵ�ȼ (�A����B��)���ľƾ��ƣ��ȹ۲쵽�������������������������������ٵ�ȼ��һ�ƾ��ơ���������Ŀ��������������������������������?
(3)װ��D�������Ǽ��鼯��ƿ���Ƿ��ռ�����H2�������IJ�������Ҫ�۲������������������������?
(4)��Ӧһ��ʱ���Bװ�ò������п��ܲ����Ĺ���������(д��ѧʽ)������������������������������ѡ����һ�֣����ʵ��֤�����Ĵ��ڣ���Ҫд����Ҫ�����������Լ��������ۡ���������������������
��.Mg + 2H2O![]() Mg(OH)2 + H2��???
Mg(OH)2 + H2��???
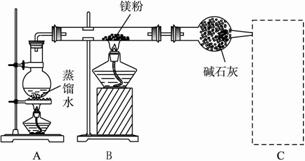
��.(1)m��x
(2)A����ˮ���ں��ž������������������Mg������Ӧ?
(3)��Dװ����Cװ�������ռ�һ�Թܵ��������壬�����Թ��ƽ��ƾ��ƻ��棬�۲챬�����Ĵ�С???
(4)Mg��MgO��Mg(OH)2?
ȡ������Ӧ��Ĺ�����Ͷ��ϡ����(��ϡ����)�У��������ݲ�����˵��þ���в���(�����������Ҳ��)
������(1)�������ܶ�С��Ӧ��������������?
(2)װ�����п������ȼ���B����ʱ��������Mg��Ӧ�γ�MgO��Ӱ��ʵ�顣?
(3)����ƿ���Ƿ��ռ���H2����ͨ������D����H2�Ĵ�������֤��?
(4)������Mg(ʣ��)��Mg(OH)2(����)��MgO��Mg(OH)2�ֽ⡳��������Mg�����ᷴӦ�ų������������͡�

 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�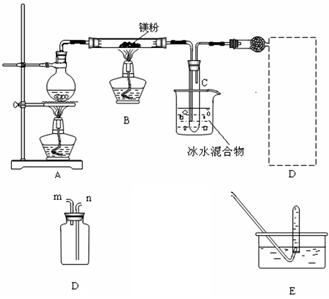
 �ȹ۲쵽���������ٵ�ȼ��һ�ƾ��ơ���������Ŀ������������������
�ȹ۲쵽���������ٵ�ȼ��һ�ƾ��ơ���������Ŀ������������������ Ӧһ��ʱ���Bװ�ò������п��ܲ����Ĺ������ʳ�MgO�⣬������M
Ӧһ��ʱ���Bװ�ò������п��ܲ����Ĺ������ʳ�MgO�⣬������M g��Mg(OH)2 ��ѡ����һ�֣����ʵ��֤�����Ĵ��ڣ���Ҫд����Ҫ�����������Լ��������ۡ�
g��Mg(OH)2 ��ѡ����һ�֣����ʵ��֤�����Ĵ��ڣ���Ҫд����Ҫ�����������Լ��������ۡ�