��Ŀ����
��2���绯ѧ��ʴ����ɸ�����ʴ����Ҫԭ��������
��2���绯ѧ��ʴ�������ⸯʴ��������ʴ��
��2�������ĸ�ʴ�ɷ�Ϊ��ѧ��ʴ�͵绯ѧ��ʴ���绯ѧ��ʴ�������ⸯʴ��������ʴ���ʴ�Ϊ�����⣻������

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�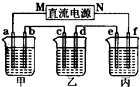 Ŀǰ��ͨ�ĵ����������Ӳ�Ҳ��Ϸֱ�Ϊ��1ԪӲ��Ϊͭо�����Ͻ�5��Ӳ��Ϊͭо��ͭ�Ͻ�1��Ӳ��ΪӲ���Ͻ���ش𣺣���Ŀ�еİٷֺ�����Ϊ����������
Ŀǰ��ͨ�ĵ����������Ӳ�Ҳ��Ϸֱ�Ϊ��1ԪӲ��Ϊͭо�����Ͻ�5��Ӳ��Ϊͭо��ͭ�Ͻ�1��Ӳ��ΪӲ���Ͻ���ش𣺣���Ŀ�еİٷֺ�����Ϊ������������1�����Ǻ�̼��Ϊ0.03%��2%��
��2����ͼ��ʾ��װ���У��ס��ҡ��������ձ��ֱ�ʢ����������Һ���缫����Һ���±���ʾ��
| �缫 | a | b | c | d | e | f |
| ʯī | ʯī | ͭ | �� | �� | ͭ | |
| ��Һ | NaCl��Һ | CuSO4��Һ | CuSO4��Һ | |||
������װ����M��Ϊֱ����Դ��
�����ڸֱ��϶�ͭ��Ӧѡ��
��3����ҵ������������Ҫ�ɷ�Al2O3?nH2O������������������ʯӢ�����ʣ�Ϊԭ����������Ϊ��ȥ��������Ʒ����������ʯӢ���ʣ�Ҫ�����顢ɸѡ�����������Ʒ�ܽ�������������������Һ�д�������д���йط�Ӧ�����ӷ���ʽ
��4����֪Ӳ���к�Cu��2.2%��5%��Mg��0.2%��3%��Mn��0.3%��1.5%��Si��0.5%��������Al��1��Ӳ�ҵ�ö����Ϊ2.20�ˣ������Ƶ�1��Ӳ��1����ö��������������Ҫ��Al2O3 90%��������Լ
Ŀǰ��ͨ�ĵ����������Ӳ�Ҳ��Ϸֱ�Ϊ��1ԪӲ��Ϊͭо�����Ͻ�5��Ӳ��Ϊͭо��ͭ�Ͻ�1��Ӳ��ΪӲ���Ͻ���ش𣺣���Ŀ�еİٷֺ�����Ϊ����������
(1)���Ǻ�̼��Ϊ0.03%��2%��___________��������������ƣ���
(2)��ͼ��ʾ��װ���У��ס��ҡ��������ձ��ֱ�ʢ����������Һ���缫����Һ���±���ʾ��
| �缫 | a | b | c | d | e | f |
| ʯī | ʯī | ͭ | �� | �� | ͭ | |
| ��Һ | NaCl��Һ | CuSO4��Һ | CuSO4��Һ |
ͨ���缫a�Ͽɲ�����ʹʪ��ĵ⻯�ص�����ֽ���������塣
������װ����M��Ϊֱ����Դ��_________������������������缫b�Ϸ����ĵ缫��ӦʽΪ__________________________________________��
�����ڸֱ��϶�ͭ��Ӧѡ��_______�ձ�����ҡ������������缫a�����ɱ�״��������2240 mLʱ�������Ͽ��ڵ缫_______������ĸ���϶�ͭ_______g��
(3)��ҵ������������Ҫ�ɷ�Al2O3��nH2O������������������ʯӢ�����ʣ�Ϊԭ����������Ϊ��ȥ��������Ʒ����������ʯӢ���ʣ�Ҫ�����顢ɸѡ�����������Ʒ�ܽ�������������������Һ�д�������д���йط�Ӧ�����ӷ���ʽ__________________________��
(4)��֪Ӳ���к�Cu��2.2%��5%��Mg��0.2%��3%��Mn��0.3%��1.5%��Si��0.5%��������Al��1��Ӳ�ҵ�ö����Ϊ2.20�ˣ������Ƶ�1��Ӳ��1����ö��������������Ҫ��Al2O3 90%��������Լ_______�֣�С�������һλ���֣���
Ŀǰ��ͨ�ĵ����������Ӳ�Ҳ��Ϸֱ�Ϊ��1ԪӲ��Ϊͭо�����Ͻ�5��Ӳ��Ϊͭо��ͭ�Ͻ�1��Ӳ��ΪӲ���Ͻ���ش𣺣���Ŀ�еİٷֺ�����Ϊ����������
(1)���Ǻ�̼��Ϊ0.03%��2%��___________��������������ƣ���
(2)��ͼ��ʾ��װ���У��ס��ҡ��������ձ��ֱ�ʢ����������Һ���缫����Һ���±���ʾ��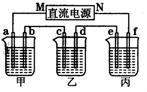
| �缫 | a[��Դ:ѧ����ZXXK] | b | c | d | e | f[��Դ:ѧ+��+��Z+X+X+K] |
| ʯī | ʯī | ͭ | �� | �� | ͭ | |
| ��Һ | NaCl��Һ | CuSO4��Һ | CuSO4��Һ | |||
������װ����M��Ϊֱ����Դ��_________������������������缫b�Ϸ����ĵ缫��ӦʽΪ__________________________________________��
�����ڸֱ��϶�ͭ��Ӧѡ��_______�ձ�����ҡ������������缫a�����ɱ�״��������2240 mLʱ�������Ͽ��ڵ缫_______������ĸ���϶�ͭ_______g��
(3)��ҵ������������Ҫ�ɷ�Al2O3��nH2O������������������ʯӢ�����ʣ�Ϊԭ����������Ϊ��ȥ��������Ʒ����������ʯӢ���ʣ�Ҫ�����顢ɸѡ�����������Ʒ�ܽ�������������������Һ�д�������д���йط�Ӧ�����ӷ���ʽ__________________________��
(4)��֪Ӳ���к�
 Cu��2.2%��5%��Mg��0.2%��3%��Mn��0.3%��1.5%��Si��0.5%��������Al��1��Ӳ�ҵ�ö����Ϊ2.20�ˣ������Ƶ�1��Ӳ��1����ö��������������Ҫ��Al2O3 90%��������Լ_______�֣�С�������һλ���֣���
Cu��2.2%��5%��Mg��0.2%��3%��Mn��0.3%��1.5%��Si��0.5%��������Al��1��Ӳ�ҵ�ö����Ϊ2.20�ˣ������Ƶ�1��Ӳ��1����ö��������������Ҫ��Al2O3 90%��������Լ_______�֣�С�������һλ���֣��� Ŀǰ��ͨ�ĵ����������Ӳ�Ҳ��Ϸֱ�Ϊ��1ԪӲ��Ϊͭо�����Ͻ�5��Ӳ��Ϊͭо��ͭ�Ͻ�1��Ӳ��ΪӲ���Ͻ���ش𣺣���Ŀ�еİٷֺ�����Ϊ����������
(1)���Ǻ�̼��Ϊ0.03%��2%��___________��������������ƣ���
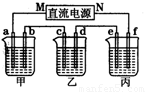
(2)��ͼ��ʾ��װ���У��ס��ҡ��������ձ��ֱ�ʢ����������Һ���缫����Һ���±���ʾ��
|
�缫 |
a[��Դ:ZXXK] |
b |
c |
d |
e |
f[��Դ:ѧ+��+��Z+X+X+K] |
|
ʯī |
ʯī |
ͭ |
�� |
�� |
ͭ |
|
|
��Һ |
NaCl��Һ |
CuSO4��Һ |
CuSO4��Һ |
ͨ���缫a�Ͽɲ�����ʹʪ��ĵ⻯�ص�����ֽ���������塣
������װ����M��Ϊֱ����Դ��_________������������������缫b�Ϸ����ĵ缫��ӦʽΪ__________________________________________��
�����ڸֱ��϶�ͭ��Ӧѡ��_______�ձ�����ҡ������������缫a�����ɱ�״��������2240 mLʱ�������Ͽ��ڵ缫_______������ĸ���϶�ͭ_______g��
(3)��ҵ������������Ҫ�ɷ�Al2O3��nH2O������������������ʯӢ�����ʣ�Ϊԭ����������Ϊ��ȥ��������Ʒ����������ʯӢ���ʣ�Ҫ�����顢ɸѡ�����������Ʒ�ܽ�������������������Һ�д�������д���йط�Ӧ�����ӷ���ʽ__________________________��
(4)��֪Ӳ���к�Cu��2.2%��5%��Mg��0.2%��3%��Mn��0.3%��1.5%��Si��0.5%��������Al��1��Ӳ�ҵ�ö����Ϊ2.20�ˣ������Ƶ�1��Ӳ��1����ö��������������Ҫ��Al2O3 90%��������Լ_______�֣�С�������һλ���֣���