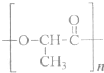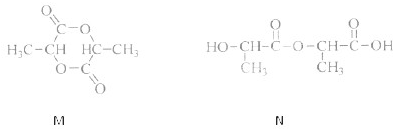��Ŀ����
��������ɸ�������ɺ����ʽ��з��ࣺ

��1��������ʾ�����ʷ���������� ��
��2����Na��K��H��O��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��Тڡ��ۺ͢��档��ÿ����дһ����ѧʽ���ɣ�
|
������� |
�� |
�� |
�� |
������ |
�⻯�� |
|
��ѧʽ |
��HCl �� |
�� ��Ba(OH)2 |
��Na2CO3 �� |
��CO2 ��Na2O |
��NH3 ��H2O |
��3������10�����ʣ���ˮ���ڿ�������ͭ˿���ܶ�����̼����������������ƣ��������ƣ���Fe(OH)3���壻��̼�����[Ca(HCO3)2]����NH3
���ڵ���ʵ��� ____________������ţ���ͬ��; ���ڷǵ���ʵ���____________��
��4����Ҫ��д�����з�Ӧ�����ӷ���ʽ��
��п��ϡ���ᷴӦ_______________________________________________________,
������������Һ��ϡ���ᷴӦ ____________________________________________,
������þ�μ�ϡ����____________________________________________________��
(1) ��״���෨ (1��)
(2) �� H2SO4��HNO3 ��NaOH��KOH ��NaNO3��KNO3 ��K2SO4��Na2SO4 (��1��)
(3) ����ʢ٢ݢޢߢ� �ǵ���ʢܢ� (��2��)
(4) ��Zn+2H+ = Zn2+ +H2 ��
��Ba2+ +2OH-+2H+ +SO42-=BaSO4�� + 2H2O
��MgO + 2H+ = Mg2+ +H2O (��2��)
��������
�����������1����״���෨��һ�ֺ�����ķ��෨�����ղ�Σ�һ��һ�����֣�����һ�ô�������Ҷ��֦���ˡ�����ͼʾ����������״ͼ���ʴ�Ϊ����״���෨��
��2����������������ȫ���������ӵĻ������H2SO4����������������ȫ��Ϊ���������ӣ���NaOH���ε������������Ϊ�������ӣ�������Ϊ������ӣ���K2SO4��
�ʴ�Ϊ����H2SO4����NaOH����K2SO4��
��3��Ba��OH��2�ĵ��뷽��ʽBa��OH��2=Ba2++2OH-���ʴ�Ϊ��Ba(OH)2=Ba2++2OH-��
��4�������̼���Ʒ�Ӧ����ˮ�Ͷ�����̼����Ӧ�����ӷ���ʽCO32-+2H+=H2O+CO2�����ʴ�Ϊ��CO32-+2H+=H2O+CO2����
���㣺�ᡢ��Ρ�������ĸ�������ϵ��

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�