��Ŀ����
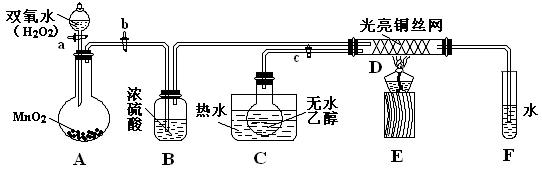
ijʵ��С��������װ�ý����Ҵ���������ʵ�飮

��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ
��2����������ˮԡ���ò���ͬ����������
��3����Ӧ����һ��ʱ����Թ�a�����ռ�����ͬ�����ʣ�������
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����
Ҫ��ȥ�����ʣ����ڻ��Һ�м���
a���Ȼ�����Һ����������b������������ c��̼��������Һ ������d�����Ȼ�̼
Ȼ����ͨ��

��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ
2Cu+O2
2CuO
| ||
2Cu+O2
2CuO
��
| ||
CH3CH2OH+CuO
CH3CHO+Cu+H2O
| �� |
CH3CH2OH+CuO
CH3CHO+Cu+H2O
��| �� |
��2����������ˮԡ���ò���ͬ����������
����
����
���ҵ���������ȴ
��ȴ
����3����Ӧ����һ��ʱ����Թ�a�����ռ�����ͬ�����ʣ�������
��ȩ���Ҵ���ˮ
��ȩ���Ҵ���ˮ
������ƿ���ռ������������Ҫ�ɷ�������
����
����4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����
����
����
��Ҫ��ȥ�����ʣ����ڻ��Һ�м���
c
c
����д��ĸ����a���Ȼ�����Һ����������b������������ c��̼��������Һ ������d�����Ȼ�̼
Ȼ����ͨ��
����
����
��������������ƣ����ɳ�ȥ����������1���Ҵ��Ĵ�������Ӧʵ���ǣ�����ͭ����������Ϊ����ͭ������ͭ���Ҵ�����Ϊ��ȩ������ͭ��������ã�
��2����ˮԡ����ˮԡ�������Dz�ͬ�ģ�
��3���������ʵķе�ߵͲ�ͬ��ȷ����õ����ʣ���Ͽ����ijɷ��Լ������ķ�Ӧȷ��ʣ�������ɷ֣�
��4����ʹ��ɫʯ����ֽ�������ᣬ̼�����ƿ��Ժ����ᷴӦ��
��2����ˮԡ����ˮԡ�������Dz�ͬ�ģ�
��3���������ʵķе�ߵͲ�ͬ��ȷ����õ����ʣ���Ͽ����ijɷ��Լ������ķ�Ӧȷ��ʣ�������ɷ֣�
��4����ʹ��ɫʯ����ֽ�������ᣬ̼�����ƿ��Ժ����ᷴӦ��
����⣺��1���Ҵ��Ĵ�������Ӧ���̣�����ͭ����������Ϊ����ͭ��2Cu+O2
2CuO������ͭ���Ҵ�����Ϊ��ȩ��CH3CH2OH+CuO
CH3CHO+Cu+H2O���ʴ�Ϊ��2Cu+O2
2CuO��CH3CH2OH+CuO
CH3CHO+Cu+H2O��
��2����������ˮԡ���ò���ͬ��������ˮԡ���������Ҵ�ƽ���������Ҵ�������������ˮԡ��Ŀ���ǽ���ȩ��ȴ�������ʴ�Ϊ�����ȣ���ȴ��
��3���Ҵ��Ĵ�����ʵ���е����ʣ��Ҵ�����ȩ��ˮ�ķе�ߵͲ�ͬ�����Թ�a�����ռ���Щ��ͬ�����ʣ������ijɷ���Ҫ�ǵ����������������μӷ�Ӧ��ʣ�����Ҫ�ǵ������ʴ�Ϊ����ȩ���Ҵ���ˮ��������
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л��������ᣬ�ĸ�ѡ����У�ֻ��̼�����ƿ��Ժ����ᷴӦ�����������ơ�ˮ�Ͷ�����̼��ʵ�����ֻ������ʵķ���������
�ʴ�Ϊ�����c������
| ||
| �� |
| ||
| �� |
��2����������ˮԡ���ò���ͬ��������ˮԡ���������Ҵ�ƽ���������Ҵ�������������ˮԡ��Ŀ���ǽ���ȩ��ȴ�������ʴ�Ϊ�����ȣ���ȴ��
��3���Ҵ��Ĵ�����ʵ���е����ʣ��Ҵ�����ȩ��ˮ�ķе�ߵͲ�ͬ�����Թ�a�����ռ���Щ��ͬ�����ʣ������ijɷ���Ҫ�ǵ����������������μӷ�Ӧ��ʣ�����Ҫ�ǵ������ʴ�Ϊ����ȩ���Ҵ���ˮ��������
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л��������ᣬ�ĸ�ѡ����У�ֻ��̼�����ƿ��Ժ����ᷴӦ�����������ơ�ˮ�Ͷ�����̼��ʵ�����ֻ������ʵķ���������
�ʴ�Ϊ�����c������
�����������漰��ѧʵ����������Լ��Ҵ��Ĵ�����ʵ��ȷ����֪ʶ�������ۺ�֪ʶ�Ŀ��飬�ۺ��Խ�ǿ���Ѷ��еȣ�

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ