��Ŀ����
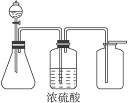
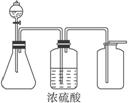
��1����ͼ��һ��ʵ������ȡ�����װ�ã����ڷ�����������ռ����塣���и�����������������װ�ý���ʵ�����_____________�����ţ���
A.MnO2��Ũ���� B.Zn��ϡ����
C.MnO2��H2O2 D.CaCO3������
��2��������������Һ���뺬�з�̪������������Һ�У���ɫ��ʧ����ͬѧ��Ϊ������Ϊ���������Ƕ�Ԫ���ᣨH2O2![]() H++
H++![]() ��������OH-����ʹ��̪��ɫ����ͬѧ��Ϊ�������������ǿ�����ԣ�����̪��������ɫ��ʧ�������һ�������е�ʵ����֤�ס�����ͬѧ�Ľ����ĸ���ȷ��
��������OH-����ʹ��̪��ɫ����ͬѧ��Ϊ�������������ǿ�����ԣ�����̪��������ɫ��ʧ�������һ�������е�ʵ����֤�ס�����ͬѧ�Ľ����ĸ���ȷ��
��3�����õ��ͻ��İ�ɫ��λ����ѧ�ɷ�ΪPbSO4�������ڣ���ѧ�ɷ�ΪPbS������˫��ˮ���ú��ֿɻָ�ԭò��������Ӧ�Ļ�ѧ����ʽΪ____________________________��
��1��CD ��2������ɫ�����Һ�м������������������Һ�������Һ���±�죬���ͬѧ�Ľ�����ȷ�������Һ����죬����ͬѧ�Ľ�����ȷ
��3��PbS+4H2O2![]() PbSO4��+4H2O
PbSO4��+4H2O
����:��1��MnO2��Ũ���ᷴӦ��Cl2����ȣ�Zn��ϡH2SO4��Ӧ��H2��Ӧ�������ſ������ռ������A��B�����ô�װ�ý���ʵ�顣
��2����ͬѧ��Ϊ����ΪH2O2������OH-��ʹ��̪��ɫ����ô���������OH-������Һ�Ƿ��ֱ�죬��֤���۵��Ƿ���ȷ��

 �ִʾ��ƪϵ�д�
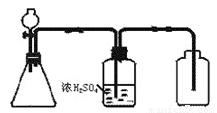
�ִʾ��ƪϵ�д���1����ͼ��һ��ʵ��������װ�ã����ڷ�����������ռ����壬���и�������������������װ�ý���ʵ����� _ ������ţ���
���������ƺ�����ڶ������̺�Ũ�����Ũ��ˮ����ʯ�Ң�ʯ��ʯ��ϡ�����п��ϡ�������������ϡ�����˫��ˮ�Ͷ�������
��2������ƿʧȥ��ǩ�����ʵ���Ũ����ͬ��̼������Һ��̼��������Һ��������Լ���
�������Լ��У���ѡ�õ�һ���Լ���_______������ţ���?
| A������������Һ | B��������Һ | C��ϡ���� | D������ʯ��ˮ |
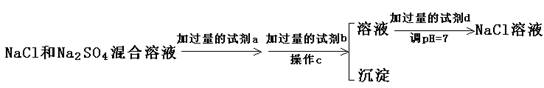
��3��ʵ������Ҫ�ô�����NaCl��Һ�����е�NaCl�����л���������Na2SO4��(NH4)2CO3�������ʵ���ȥ���ʣ����ش��������⣺
�ٳ�ȥ(NH4)2CO3�ü��Ⱥû��Ǽ�ǿ������Ⱥã���ѡ����� ����������
��?
�������dz�ȥSO42����ʵ�鷽����

������a�Լ��� ��C��������ʹ�õIJ��������������� ��֤��SO42���Ѿ�������ȫ�ķ�����
 ��
��