��Ŀ����
2012��3��22���ǵڶ�ʮ�조����ˮ�ա�������ˮ��Դ����������
��1��ClO2��Cl2����ԭ���ﶼΪCl-���������г��õ��������������ĵ����ʵ�������������ʱ��ClO2������Ч����Cl2��______����
��2������ˮ�����ø������ƣ�Na2FeO4��ǿ������������ˮ�ʣ�����������������ɱ������ͬʱ����ˮ���õ�ԭ��______��
��3��ij��ɫ��ˮ�п��ܺ���Fe2+��Al3+��Na+��NO3-��CO32-��SO42-�����еļ��֣�Ϊ������ɷ֣��ֱ�ȡ��ˮ��Ʒ100mL������������ʵ�飬��������й�������ͼ��ʾ��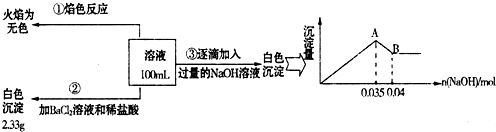
�������ͼ�ش��������⣺
��ʵ����������1.0mol/L��NaOH��Һ100mL�������������˲�������������ƽ����Ͳ��ҩ�ס��ձ�����ͷ�ιܣ���ȱ�ٵ�����Ϊ______��
��ʵ����г�������A��B��������������Ӧ�����ӷ���ʽΪ______��
����ȷ��NO3-�Ƿ���ڣ�______������ڡ����������ڡ���ȷ�������������ڣ��Լ���c(NO3-)=______���������ڣ����ʲ������𣩣�
��1��ClO2��Cl2����ԭ���ﶼΪCl-���������г��õ��������������ĵ����ʵ�������������ʱ��ClO2������Ч����Cl2��______����
��2������ˮ�����ø������ƣ�Na2FeO4��ǿ������������ˮ�ʣ�����������������ɱ������ͬʱ����ˮ���õ�ԭ��______��
��3��ij��ɫ��ˮ�п��ܺ���Fe2+��Al3+��Na+��NO3-��CO32-��SO42-�����еļ��֣�Ϊ������ɷ֣��ֱ�ȡ��ˮ��Ʒ100mL������������ʵ�飬��������й�������ͼ��ʾ��

�������ͼ�ش��������⣺
��ʵ����������1.0mol/L��NaOH��Һ100mL�������������˲�������������ƽ����Ͳ��ҩ�ס��ձ�����ͷ�ιܣ���ȱ�ٵ�����Ϊ______��
��ʵ����г�������A��B��������������Ӧ�����ӷ���ʽΪ______��
����ȷ��NO3-�Ƿ���ڣ�______������ڡ����������ڡ���ȷ�������������ڣ��Լ���c(NO3-)=______���������ڣ����ʲ������𣩣�
��1��ClO2��Cl2�Ļ�ԭ���ﶼΪCl-��ÿĦ��Cl2�õ�2mol���ӣ���ÿĦ��ClO2�õ�5mol���ӣ�������Cl2�����ʵ�����ClO2��2.5�����ʴ�Ϊ��2.5��
��2���������ƾ���ǿ�����ԣ�������ɱ����������ԭ����Fe3+��Fe3+����ˮ����������������������ˮ�е����ʣ���������ȥˮ�е�������ﵽ��ˮ��Ŀ�ģ�
�ʴ�Ϊ��FeO42-��ǿ�������ԣ���ɱ����������������ԭΪFe3+��Fe3+����ˮ����������������������ˮ�е����ʣ��ﵽ��ˮ��Ŀ�ģ�
��3������1.0mol/L��NaOH��Һ100mL������ʵ�鲽��������������������������ƽ����Ͳ��ҩ�ס��ձ�����ͷ�ιܡ�100mL����ƿ���ʴ�Ϊ��100mL����ƿ��
��ʵ�����A��B������Al��OH��3��OH-������Ӧ���䷽��ʽΪ��Al��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
�۸���ʵ���ȷ����Na+������ʵ���ȷ����SO42-������ʵ���ȷ����Al3+��Fe2+����ΪCO32-��Al3+���ܹ��棬������CO32-������Һ�д��ڵ�����Ϊ��Al3+��SO42-����֪���ᱵ����Ϊ2.33g����n��SO42-��=
=0.01mol��
����ͼ���֪��Al��OH��3��OH-Ϊ��n��OH-��=0.005mol��
Al��OH��3 +OH-=AlO2-+2H2O Fe2++2OH-=Fe��OH��2��
n��Al3+�� 0.005moln��Fe2+�� 0.035-3n��Al3+��
����n��Al3+��=0.005mol��n��Fe2+��=0.01mol ������Һ��Fe2+��Al3+�����������SO42-��������ɲ���ȣ���˴���NO3-��
��NO3-���ʵ���Ϊnmol�����ݵ���غ�ã�3n��Al3+��+2n��Fe2+��=2n��SO42-��+n��NO3-������n��NO3-��=0.015mol������c��NO3-���T
=0.15mol/L��
�ʴ�Ϊ�����ڣ�0.15mol/L��
��2���������ƾ���ǿ�����ԣ�������ɱ����������ԭ����Fe3+��Fe3+����ˮ����������������������ˮ�е����ʣ���������ȥˮ�е�������ﵽ��ˮ��Ŀ�ģ�
�ʴ�Ϊ��FeO42-��ǿ�������ԣ���ɱ����������������ԭΪFe3+��Fe3+����ˮ����������������������ˮ�е����ʣ��ﵽ��ˮ��Ŀ�ģ�
��3������1.0mol/L��NaOH��Һ100mL������ʵ�鲽��������������������������ƽ����Ͳ��ҩ�ס��ձ�����ͷ�ιܡ�100mL����ƿ���ʴ�Ϊ��100mL����ƿ��
��ʵ�����A��B������Al��OH��3��OH-������Ӧ���䷽��ʽΪ��Al��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
�۸���ʵ���ȷ����Na+������ʵ���ȷ����SO42-������ʵ���ȷ����Al3+��Fe2+����ΪCO32-��Al3+���ܹ��棬������CO32-������Һ�д��ڵ�����Ϊ��Al3+��SO42-����֪���ᱵ����Ϊ2.33g����n��SO42-��=
| 2.33g |
| 233g/mol |
����ͼ���֪��Al��OH��3��OH-Ϊ��n��OH-��=0.005mol��
Al��OH��3 +OH-=AlO2-+2H2O Fe2++2OH-=Fe��OH��2��
n��Al3+�� 0.005moln��Fe2+�� 0.035-3n��Al3+��
����n��Al3+��=0.005mol��n��Fe2+��=0.01mol ������Һ��Fe2+��Al3+�����������SO42-��������ɲ���ȣ���˴���NO3-��
��NO3-���ʵ���Ϊnmol�����ݵ���غ�ã�3n��Al3+��+2n��Fe2+��=2n��SO42-��+n��NO3-������n��NO3-��=0.015mol������c��NO3-���T
| 0.015mol |
| 0.1L |
�ʴ�Ϊ�����ڣ�0.15mol/L��

��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ