��Ŀ����
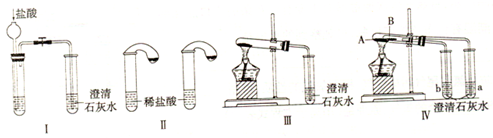
ij����С��Ϊ�˼���̼���ƺ�̼���������ְ�ɫ���壬�ò�ͬ�ķ���������ͼ��ʾʵ�顣

(1)ֻ����ͼI��II��ʾʵ�飬�ܹ��ﵽʵ��Ŀ���ǣ���װ����ţ�_____________________��
(2)ͼIII��IV��ʾʵ����ܼ������������ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ____________________����ʵ��III�� �ȣ�ʵ��IV���ŵ��ǣ���ѡ����ţ�____________________��
A��IV��III����
B��IV��III��ȫ
C��IV��III�������
D��IV����������һ��װ��ͬʱ���������Ա�ʵ�飬��III����
(3)����ʵ��IV��֤̼���ƺ�̼�����Ƶ��ȶ��ԣ����Թ�B��װ��Ĺ��������_________________________��
(4)��̼��������Һ�����ʯ��ˮ��ϲ���ַ�Ӧ��
�ٵ�ʯ��ˮ����ʱ�������ӷ���ʽΪ_______________________��
�ڵ�̼�������������������ʵ���֮��Ϊ2:1ʱ��������Һ�����ʵĻ�ѧʽΪ________________________��
�����ʵ�����������Һ�����ʵ�������____________________________��
(2)ͼIII��IV��ʾʵ����ܼ������������ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ____________________����ʵ��III�� �ȣ�ʵ��IV���ŵ��ǣ���ѡ����ţ�____________________��
A��IV��III����
B��IV��III��ȫ
C��IV��III�������
D��IV����������һ��װ��ͬʱ���������Ա�ʵ�飬��III����
(3)����ʵ��IV��֤̼���ƺ�̼�����Ƶ��ȶ��ԣ����Թ�B��װ��Ĺ��������_________________________��
(4)��̼��������Һ�����ʯ��ˮ��ϲ���ַ�Ӧ��
�ٵ�ʯ��ˮ����ʱ�������ӷ���ʽΪ_______________________��
�ڵ�̼�������������������ʵ���֮��Ϊ2:1ʱ��������Һ�����ʵĻ�ѧʽΪ________________________��
�����ʵ�����������Һ�����ʵ�������____________________________��
(1)II
(2) ��CO2+Ca(OH)2=CaCO3��+H2O��D
��CO2+Ca(OH)2=CaCO3��+H2O��D
(3)NaHCO3
(4)��Ca2++OH-+HCO3-=CaCO3��+H2O ��Na2CO3ȡ�����ϲ���Һ���Թ��У����������Ȼ�����Һ�������а�ɫ�������ɣ���֤����Һ�к���CO32-
(2)
 ��CO2+Ca(OH)2=CaCO3��+H2O��D
��CO2+Ca(OH)2=CaCO3��+H2O��D (3)NaHCO3
(4)��Ca2++OH-+HCO3-=CaCO3��+H2O ��Na2CO3ȡ�����ϲ���Һ���Թ��У����������Ȼ�����Һ�������а�ɫ�������ɣ���֤����Һ�к���CO32-

��ϰ��ϵ�д�
�����Ŀ
I����ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ����ѧʵ���ڻ�ѧѧϰ�о�����Ҫ�����á�
���й���ʵ�����������ȷ���� ����ѡ����ĸ��
| A���������Ż�ʱ��Ӧ�����ô���ˮ���� |
| B����������մ��Ƥ���������ϣ�Ӧ������ŨNaOH��ϴ |
| C�������ᾧʱӦ����Һ���ɺ���ֹͣ���� |
| D������ʱ��Ӧʹ�¶ȼ�ˮ��������Һ���� |
F. ʹ������ƿǰ������Ƿ�©ˮ
G��������Һʱ������ʱ���ӿ̶��߹۲�Һ��
H����ɫ��Ӧʵ��ʱ��ÿ����һ��ʵ�飬��˿Ӧ��մ��ϡ������ڻ��������յ���ɫʱ��������һ��ʵ�顣
II��ij����С��Ϊ�˼���̼���ƺ�̼���������ְ�ɫ���壬�����˲�ͬ��ʵ�鷽����(װ������ͼ��ʾ)

(1)������ͼI������ʾʵ�飬�ܹ��ﵽʵ��Ŀ�ĵ��� (ѡ��װ�����)��
(2)ͼIII��ʾʵ���ܹ�����̼���ƺ�̼���������������ʣ���д���Թ�C�з�����Ӧ�Ļ�ѧ����ʽ ��
(3)ͼ�������ܼ���̼���ƺ�̼�����ƣ�ͬʱ������֤̼���ƺ�̼�����Ƶ��ȶ��ԣ����Թ�B��װ�������Ϊ ��ʵ������е�����Ϊ ��