��Ŀ����
2008��5��12���Ĵ��봨�����ش�����Ϊ��ֹ�ֺ��߲�ʹ���˴����ĸ�������Һ����NaClO��Һ��ijѧϰС�������Һ��������(NaClO)���Ʊ������ʽ����о���
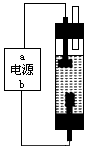
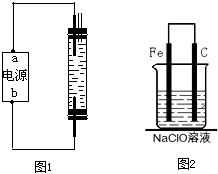
��һ����ͬѧ��ʯī�ͱ���ʳ��ˮ�����ͼ�Ʊ�����Һ( NaClO ��Һ���ķ�������ͨ��ʱ����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ___ ��Ϊʹ���ɵ�Cl2��ȫ�����գ����Դ��a��ӦΪ___�������������������Һ������NaClO�����ӷ���ʽΪ____��
��һ����ͬѧ��ʯī�ͱ���ʳ��ˮ�����ͼ�Ʊ�����Һ( NaClO ��Һ���ķ�������ͨ��ʱ����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ___ ��Ϊʹ���ɵ�Cl2��ȫ�����գ����Դ��a��ӦΪ___�������������������Һ������NaClO�����ӷ���ʽΪ____��
��������ͬѧ��ij���в�ѯ��ijƷ������Һ��װ˵���IJ������ݣ���Ҫ��Ч�ɷ�Ϊ�������ƣ���Ч�Ⱥ���8 000�� 10 000 mg/L�������ڸ���Ҿ���Ʒ���;ߡ���֯����ȵ��������Բ�ɫ֯���������ɫ���á���Ʒ���ܷ⣬�������������档
(1)����ʱ������ø�����Һ(NaClO)��pH>7����ԭ��Ϊ�������ӷ���ʽ��ʾ��___��
(2)������Һ�����еĻ�ѧ������____������ţ���
A��ǿ������
B��ǿ��ԭ��
C�����ȶ���
D��Ư����
(3)�Ӹ�����Һ�ı���Ҫ�������������ʧЧ����Ҫԭ���ǣ��û�ѧ����ʽ��ʾ��___��
(1)����ʱ������ø�����Һ(NaClO)��pH>7����ԭ��Ϊ�������ӷ���ʽ��ʾ��___��
(2)������Һ�����еĻ�ѧ������____������ţ���
A��ǿ������
B��ǿ��ԭ��
C�����ȶ���
D��Ư����
(3)�Ӹ�����Һ�ı���Ҫ�������������ʧЧ����Ҫԭ���ǣ��û�ѧ����ʽ��ʾ��___��
��һ�� 2NaCl+2H2O 2NaOH+H2��+Cl2�� ���� ��Cl2+2OH-=Cl-+ClO-+H2O
2NaOH+H2��+Cl2�� ���� ��Cl2+2OH-=Cl-+ClO-+H2O
������(1)ClO-+H2O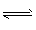 HClO+OH-
HClO+OH-
(2) AD
(3)2NaClO+CO2+H2O=Na2CO3+2HClO
 2NaOH+H2��+Cl2�� ���� ��Cl2+2OH-=Cl-+ClO-+H2O
2NaOH+H2��+Cl2�� ���� ��Cl2+2OH-=Cl-+ClO-+H2O ������(1)ClO-+H2O
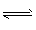 HClO+OH-
HClO+OH- (2) AD
(3)2NaClO+CO2+H2O=Na2CO3+2HClO

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��2010?��̨һģ��2008��5��12���ҹ��Ĵ��봨�����ش����Ϊ��ֹ�ڴ���֮���߲����У�ȫ�����������������˴����ĸ�������Һ����NaClO��Һ��ijУ̽����ѧϰС�������Һ�������ƣ�NaClO�����Ʊ������ʵȽ�����̽����
��2010?��̨һģ��2008��5��12���ҹ��Ĵ��봨�����ش����Ϊ��ֹ�ڴ���֮���߲����У�ȫ�����������������˴����ĸ�������Һ����NaClO��Һ��ijУ̽����ѧϰС�������Һ�������ƣ�NaClO�����Ʊ������ʵȽ�����̽���� �������ϣ���1��2007��11��26�գ��ҹ��״�����̽��̵�һ������ͼ��������������к��зḻ��3He���º����������̲��ŷḻ�Ľ��������Դ�ʹ����Ķ������衢����ȣ� ��2��2008��5��12���Ĵ�����ǿ�ҵ��𣮵��������ڵ������ʯ���ѡ������ѳ��ڻ������������������ͷų���������֪������ı���������ʯ�����������ʯ��Ҫ�ɷֶ��ǹ����Σ���������������ǣ�������
�������ϣ���1��2007��11��26�գ��ҹ��״�����̽��̵�һ������ͼ��������������к��зḻ��3He���º����������̲��ŷḻ�Ľ��������Դ�ʹ����Ķ������衢����ȣ� ��2��2008��5��12���Ĵ�����ǿ�ҵ��𣮵��������ڵ������ʯ���ѡ������ѳ��ڻ������������������ͷų���������֪������ı���������ʯ�����������ʯ��Ҫ�ɷֶ��ǹ����Σ���������������ǣ�������