��Ŀ����
(12��)���������ַ��������Ƶð�ɫ��Fe(OH)2������
����һ���ò���Fe3+��FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
��1������������������������FeSO4��Һʱ�������ϡH2SO4��______��
��2����ȥ����ˮ���ܽ��O2������____________�ķ�����
��3�����ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ�����ټ���NaOH��Һ������������������_______________________________��
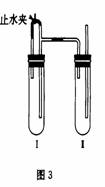
������������ͼ��ʾװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���
��1�����Թܹ��������Լ���_______________��
��2�����Թܢ��������Լ���_______________��
��3��Ϊ���Ƶð�ɫFe(OH)2���������Թܢ�͢��м����Լ�����ֹˮ�У��������Ӻ��ʵ�鲽����_________________________________��
��4���������ɵ�Fe(OH)2�����ܽϳ�ʱ�䱣�ְ�ɫ����������________________________________________________________________��
����һ��1����м ��2�����
��3���������ɵ�Fe(OH)2�����Ӵ�O2
����������1��ϡ���ᣬ��м
��2��NaOH��Һ
��3�������Թܢ���ڴ��ų��������Ĵ��ȡ����ų��������Ĵ���ʱ���ټн�ֹˮ��
�Թܢ��з�Ӧ���ɵ������������Թܢ���Թܢ���������������
����:



 2 mol A��2 mol B������������2 L�ܱ������У��������·�Ӧ��3A(g)��B(g)
2 mol A��2 mol B������������2 L�ܱ������У��������·�Ӧ��3A(g)��B(g) 2C(g)��2D(g),2����ĩ��Ӧ�ﵽƽ��״̬����
2C(g)��2D(g),2����ĩ��Ӧ�ﵽƽ��״̬����