��Ŀ����
ʵ���ǻ�ѧ�о���һ����Ҫ�ֶΣ�������ͼ��ʾA~G���������������Ҫ����ա�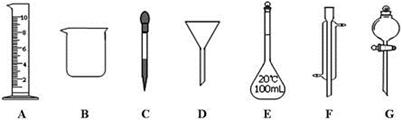
��1��д���������ƣ�E ��F
��2������ʵ��������õ�����G���� ��ѡ������ѡ��ı����ĸ����
a������ˮ��CC14�Ļ����
b������ˮ�;ƾ��Ļ����
c������ˮ����ɰ�Ļ����
��3��ʵ��������100mL 0.5mol/L��������Һ��
�����й�������E��ʹ�÷����У���ȷ���� ��ѡ������ѡ��ı����ĸ����
a��ʹ��ǰӦ����Ƿ�©Һ b��ʹ��ǰ������
c�������������ʷ�Ӧ���ܽ������ d������Һ��ֱ��ת�Ƶ�����ƿ��
����Ҫ10mol/L��Ũ���� mL��ȡ�ø��������ʱ����Ҫ�õ����������е�A�� ��ѡ�������ı����ĸ����
��1������ƿ�������ܣ�2��a ��3���� ac �� 5����5.0���� C
���������������1�����������Ľṹ�ص��ж����������ƣ�EΪ����ƿ��FΪ�����ܣ���2��GΪ��Һ©���������ڷ��뻥�����ܵ�Һ�����ѡa����3����EΪ����ƿ��ֻ���ڳ�����ʹ�ã���ֻ������������Һ������������;����ʹ��ǰҪ����Ƿ�©ˮ����Ϊ��ac���ڸ���ϡ�Ͷ��ɼ�������Ũ����������0.1L��0.5mol/L=10moL/L��V��V=0.005L����5.0mL��ȡ�ø��������ʱ����Ҫ�õ����������е���Ͳ�ͽ�ͷ�ιܣ����н�ͷ�ι����ڶ��ݣ���Ϊ��5.0��C��
���㣺���黯ѧʵ������������漰����������ʶ�����ʵķ��뼰��Һ�����ơ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����ʵ�������ȷ����(����)
| A���ýྻ�IJ�����պȡ��Һ������ʪ���pH��ֽ�ϲⶨ��ҺpH |
| B���������õĹ���ֱ�Ӽ�������ƿ�У���ˮ�ܽⲢϡ�����̶ȣ����Ƴ�һ�����ʵ���Ũ�ȵ���Һ |
| C�����ӵ�ˮ�з����I2���ɽ�������CCl4�����ˮ�����÷ֲ���Һ |
| D��Ϊ��С�к͵ζ�����ƿ����ϴ������ɺ����ʹ�� |
����ʵ��װ�á��Լ�ѡ�û������ȷ����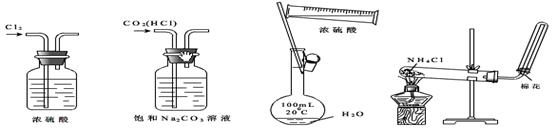
| A������Cl2 | B����ȥCO2�е�HCl | C��ϡ��Ũ���� | D����ȡ�������� |
���и���ʵ��������������ó��Ľ�����ȷ����
| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | ����Һ�м���Na2CO3��Һ | ��Һ����� | ���ԣ����ӣ� HCO3- |
| B | ���Ҵ��м���ŨH2SO4�����ȣ���Һ��ڣ�������������ͨ������KMnO4��Һ | KMnO4��Һ ��ɫ | ����������ϩ |
| C | ����ҺX�еμ�NaOHϡ��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� | ��ֽ������ | ��ҺX����NH4+ |
| D | ��ʪ��ĵ��۵⻯����ֽ��������Y | ��ֽ���� | ����Y��Cl2 |
����ʵ�������ʵ�������ȷ����
A��ʵ��������ͼ��ʾװ����ȡ��������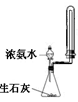 |
| B��ʵ������������ʱ���������뱽��Ϻ��ٵμ�Ũ���� |
| C��ʵ�������屽ʱ��������Һ���Ϻ�ӵ�����˿�ķ�Ӧ������ |
| D�������ƽᾧˮ�������ʯ�ҹ�����ȡ���� |
���в������ܴﵽĿ�ĵ���(����)
| ѡ�� | Ŀ�� | ���� |
| A | ����100 mL 1��0 mol��L��1CuSO4��Һ | ��25 g CuSO4��5H2O ����100 mL����ˮ�� |
| B | ��ȥKNO3 ������NaCl | ��������Ƴ��ȵı��� ��Һ����ȴ�ᾧ������ |
| C | ����Һ�н�MnO4�� ��ȫת��ΪMn2�� | ������KMnO4��Һ �еμ�H2O2��Һ����ɫ��ʧ |
| D | ȷ��NaCl��Һ���� �����Na2CO3 | ȡ������Һ�μ�CaCl2 ��Һ���۲��Ƿ���ְ�ɫ���� |
����ʵ��װ�������ȷ�����ܴﵽĿ�ĵ���(˫ѡ) (����)��
| A��ʵ����װ�õ������� |
| B��ʵ��ⶨδ֪�����Ũ�� |
| C��ʵ�����ȡ���۲�Fe(OH)2���� |
| D��ʵ���������һ�����ʵ���Ũ�ȵ�ϡ������Һ |

