��Ŀ����
 ijѧУ�����һ��ʵ���Σ���ѧ���ӷ�
ijѧУ�����һ��ʵ���Σ���ѧ���ӷ�
�ɸɵ�ػ���̼����пƤ��![]() ��
��![]() ��
��![]() �����ʣ�
�����ʣ�
����ʵ��������£���ش��й����⡣
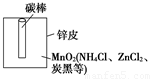
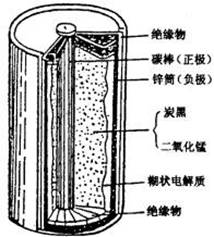
��1���йظɵ�صĻ����������ԭ������ͼ�Ǹɵ�ص�
��������ͼ���ɵ�ع���ʱ�����ϵĵ缫��Ӧʽ���� ��![]()
�������dz�ȥ�����ϵIJ����������Mn2O3���÷�Ӧ�Ļ�ѧ����ʽ�� ��
пƤ��̼���Ļ��ա���ǯ�Ӻͼ��ӿ����յĸɵ�ص�пͲ����пƤ��̼��ȡ����������ˢˢϴ�ɾ���������ڵĺ�ɫ��ĩ����С�ձ��С�
����李��Ȼ�п����ȡ������ͷ��롣
��δӺ�ɫ��ĩ����ȡ![]() ��
��![]() �Ⱦ���Ļ���д����Ҫ��ʵ�鲽�衣
�Ⱦ���Ļ���д����Ҫ��ʵ�鲽�衣
��Ƽ�ʵ��֤�����þ����к���![]() ��
��![]() [һֱ
[һֱ![]() �����������������ܽ��ڰ�ˮ]��������д����ʵ�鱨�档
�����������������ܽ��ڰ�ˮ]��������д����ʵ�鱨�档
| ʵ�鲽�� | ʵ������ | ʵ����� | �й����ӷ���ʽ |
| ��a�� | |||
| (b) | (c) | ˵���� | (d) |
| (e) | (f) | ˵���� |
(a) ,(b) ,(c) ,(d) ,(e) ,(f) ,
��4�����ʣ��ĺ�ɫ��������Ҫ�ɷ���![]() ������̿�ں��л���ȡ��������յķ�����ȥ���ʣ���ʵ������Ҫ�õ�����Ҫ�������ƾ������У�д����2�м��ɣ� ��
������̿�ں��л���ȡ��������յķ�����ȥ���ʣ���ʵ������Ҫ�õ�����Ҫ�������ƾ������У�д����2�м��ɣ� ��
��1��Zn��2e-��Zn2����2MnO2+H2= Mn2O3+H2O����3�����ܽ⡢���ˡ��������ᾧ����(a) ȡ����������������ˮ�����Һ��(b) ȡ(a)��Һ��������������NaOH��Һ�����(c)����ʹ��ֽ�������������(d) NH4����OH��![]() NH3����H2O(e) ȡ(a)��Һ��������������NaOH��Һ�����ɰ�ɫ�������������ֳ����֣�һ�ݼ�����������Һ������ ����һ�ݼӰ�ˮ������(f)���ݳ������ܣ�
NH3����H2O(e) ȡ(a)��Һ��������������NaOH��Һ�����ɰ�ɫ�������������ֳ����֣�һ�ݼ�����������Һ������ ����һ�ݼӰ�ˮ������(f)���ݳ������ܣ�
����:
���⿼��ʵ��ѧ����ʵ��̽����������1���ڸɵ���У�п����ʧ���ӣ�Ϊ��صĸ������缫��ӦʽΪ��Zn��2e-��Zn2��������ΪNH4Cl�õ����ӣ������ĵ缫��ӦΪ��2NH4����2e����2NH3����H2����MnO2��������ԭΪMn2O3,��Ӧ�Ļ�ѧ����ʽΪ��2MnO2+H2= Mn2O3+H2O����3���ٻ�����к��в��������С�ձ��м���һ����������ˮ����ֽ��裬Ȼ����ˣ�����Һ�����������У����������ᾧ����Ϊ֤�������е����ӣ���ȡ����������������ˮ�����Һ��ȡ������Һ����������NaOH��Һ����ȣ���ʪ��ĺ�ʯ����ֽ�����Թܿڣ��ɹ۲쵽��ֽ���� �й����ӷ���ʽ��NH4����OH��![]() NH3����H2O ������������п����������������ܽ��ڰ�ˮ����ȡԭ��Һ�������Թ��У���������������Һ�����ɰ�ɫ�������������ֳ����֣�һ�ݼ�����������Һ������ ����һ�ݼӰ�ˮ���������ɼ������ݰ�ɫ�������ܽ⣬˵����Һ�к���Zn2+����4��Ҫͨ�����ȷ���ȥ������Ҫ�õ��ƾ��ơ��������������ȡ�
NH3����H2O ������������п����������������ܽ��ڰ�ˮ����ȡԭ��Һ�������Թ��У���������������Һ�����ɰ�ɫ�������������ֳ����֣�һ�ݼ�����������Һ������ ����һ�ݼӰ�ˮ���������ɼ������ݰ�ɫ�������ܽ⣬˵����Һ�к���Zn2+����4��Ҫͨ�����ȷ���ȥ������Ҫ�õ��ƾ��ơ��������������ȡ�